Chemical synthesis of Nd2Fe14B/Fe-Co nanocomposite with high magnetic energy product
- PMID: 35495536
- PMCID: PMC9041725
- DOI: 10.1039/d1ra03760a
Chemical synthesis of Nd2Fe14B/Fe-Co nanocomposite with high magnetic energy product
Abstract
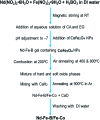
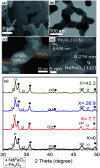
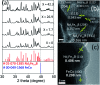
Nd2Fe14B is one of the most popular permanent magnets (PMs) possessing the best energy product (BH)max among the common PM materials. However, exchange-coupled nanocomposite magnets fabricated by embedding nanostructures of soft-phase magnetic materials into a hard-phase magnetic matrix manifest higher remanence and a higher energy product. Here we present the fabrication of exchange coupled Nd2Fe14B/Fe-Co magnetic nanocomposites using gel-combustion and diffusion-reduction processes. Pre-fabricated CoFe2O4 nanoparticles (NPs) of ∼5 nm diameter were incorporated into a Nd-Fe-B oxide matrix during its synthesis by gel-combustion. The obtained mixed oxide was further processed with oxidative annealing at 800 °C for 2 h and reductive annealing at 900 °C for 2 h to form a Nd2Fe14B/Fe-Co nanocomposite. Nanocomposites with different mol% of soft-phase were prepared and characterized by X-ray diffraction (XRD), transmission electron microscopy (TEM) and physical property measurement system (PPMS) to study their crystalline phase, morphology and magnetic behavior. Addition of 7.7 mol% of soft-phase was found to be optimum, producing a coercivity (H c) of 5.6 kOe and remanence (M r) of 54 emu g-1 in the nanocomposite.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources

