The Cell as Matter: Connecting Molecular Biology to Cellular Functions
- PMID: 35495565
- PMCID: PMC9053450
- DOI: 10.1016/j.matt.2021.03.013
The Cell as Matter: Connecting Molecular Biology to Cellular Functions
Abstract
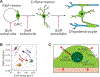
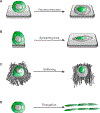
Viewing cell as matter to understand the intracellular biomolecular processes and multicellular tissue behavior represents an emerging research area at the interface of physics and biology. Cellular material displays various physical and mechanical properties, which can strongly affect both intracellular and multicellular biological events. This review provides a summary of how cells, as matter, connect molecular biology to cellular and multicellular scale functions. As an impact in molecular biology, we review recent progresses in utilizing cellular material properties to direct cell fate decisions in the communities of immune cells, neurons, stem cells, and cancer cells. Finally, we provide an outlook on how to integrate cellular material properties in developing biophysical methods for engineered living systems, regenerative medicine, and disease treatments.
Conflict of interest statement
DECLARATION OF INTERESTS: The authors declare no competing interests.
Figures



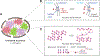
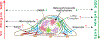


References
-
- Baluška F, Volkmann D & Barlow PW Cell bodies in a cage. Nature 428, 371–371 (2004). - PubMed
-
- Mazzarello P A unifying concept: the history of cell theory. Nature Cell Biology 1, E13–E15 (1999). - PubMed
-
- Alberts B et al. Molecular Biology of the Cell. third addition. (1994).
-
- Ho B, Baryshnikova A & Brown GW Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell systems 6, 192–205. e193 (2018). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
