Oral Immune Priming Treatment Alters Microbiome Composition in the Red Flour Beetle Tribolium castaneum
- PMID: 35495655
- PMCID: PMC9043903
- DOI: 10.3389/fmicb.2022.793143
Oral Immune Priming Treatment Alters Microbiome Composition in the Red Flour Beetle Tribolium castaneum
Abstract
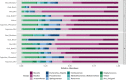
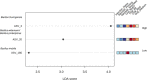
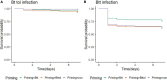
It is now well-established that the microbiome is relevant for many of an organism's properties and that its composition reacts dynamically to various conditions. The microbiome interacts with host immunity and can play important roles in the defenses against pathogens. In invertebrates, immune priming, that is, improved survival upon secondary exposure to a previously encountered pathogen, can be dependent upon the presence of the gut microbiome. However, it is currently unknown whether the microbiome changes upon priming treatment. We here addressed this question in a well-established model for immune priming, the red flour beetle Tribolium castaneum exposed to the entomopathogenic bacterium Bacillus thuringiensis (Bt). After priming treatments, the microbiota composition of beetle larvae was assessed by deep sequencing of the V1-V2 region of the bacterial 16S rRNA gene. We compared the effect of two established routes of priming treatments in this system: injection priming with heat-killed Bt and oral priming via ingestion of filtered sterilized bacterial spore culture supernatants. For oral priming, we used several strains of Bt known to vary in their ability to induce priming. Our study revealed changes in microbiome composition following the oral priming treatment with two different strains of Bt, only one of which (Bt tenebrionis, Btt) is known to lead to improved survival. In contrast, injection priming treatment with the same bacterial strain did not result in microbiome changes. Combined with the previous results indicating that oral priming with Btt depends on the larval microbiome, this suggests that certain members of the microbiome could be involved in forming an oral priming response in the red flour beetle.
Keywords: Bacillus; Tribolium castaneum; bacteria; infection; injection priming; insect immunity; microbiome; oral immune priming.
Copyright © 2022 Korša, Lo, Gandhi, Bang and Kurtz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Specificity of oral immune priming in the red flour beetle Tribolium castaneum.Biol Lett. 2017 Dec;13(12):20170632. doi: 10.1098/rsbl.2017.0632. Biol Lett. 2017. PMID: 29237813 Free PMC article.
-
Oral immune priming with Bacillus thuringiensis induces a shift in the gene expression of Tribolium castaneum larvae.BMC Genomics. 2017 Apr 26;18(1):329. doi: 10.1186/s12864-017-3705-7. BMC Genomics. 2017. PMID: 28446171 Free PMC article.
-
Microbiota Plays a Role in Oral Immune Priming in Tribolium castaneum.Front Microbiol. 2016 Jan 6;6:1383. doi: 10.3389/fmicb.2015.01383. eCollection 2015. Front Microbiol. 2016. PMID: 26779124 Free PMC article.
-
Immune priming in arthropods: an update focusing on the red flour beetle.Zoology (Jena). 2016 Aug;119(4):254-61. doi: 10.1016/j.zool.2016.03.006. Epub 2016 Mar 24. Zoology (Jena). 2016. PMID: 27350318 Review.
-
Red flour beetle (Tribolium castaneum): From population genetics to functional genomics.Vet World. 2018 Aug;11(8):1043-1046. doi: 10.14202/vetworld.2018.1043-1046. Epub 2018 Aug 2. Vet World. 2018. PMID: 30250361 Free PMC article. Review.
Cited by
-
Shotgun Metagenome Analysis of Two Schizaphis graminum Biotypes over Time With and Without Carried Cereal Yellow Dwarf Virus.Insects. 2025 May 23;16(6):554. doi: 10.3390/insects16060554. Insects. 2025. PMID: 40558984 Free PMC article.
-
Experimental evolution of a pathogen confronted with innate immune memory increases variation in virulence.PLoS Pathog. 2025 Jun 18;21(6):e1012839. doi: 10.1371/journal.ppat.1012839. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40532126 Free PMC article.
-
Differential proteome profiling of bacterial culture supernatants reveals candidates for the induction of oral immune priming in the red flour beetle.Biol Lett. 2023 Nov;19(11):20230322. doi: 10.1098/rsbl.2023.0322. Epub 2023 Nov 1. Biol Lett. 2023. PMID: 37909056 Free PMC article.
-
Visualizing Oral Infection Dynamics of Beauveria bassiana in the Gut of Tribolium castaneum.J Fungi (Basel). 2025 Jan 28;11(2):101. doi: 10.3390/jof11020101. J Fungi (Basel). 2025. PMID: 39997394 Free PMC article.
-
Resources Modulate Developmental Shifts but Not Infection Tolerance Upon Co-Infection in an Insect System.Mol Ecol. 2025 Mar 20:e17726. doi: 10.1111/mec.17726. Online ahead of print. Mol Ecol. 2025. PMID: 40109235
References
LinkOut - more resources
Full Text Sources

