Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future
- PMID: 35495689
- PMCID: PMC9048899
- DOI: 10.3389/fmicb.2022.825828
Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future
Abstract
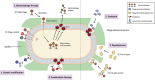
Biofilms are complex microbial microcolonies consisting of planktonic and dormant bacteria bound to a surface. The bacterial cells within the biofilm are embedded within the extracellular polymeric substance (EPS) consisting mainly of exopolysaccharides, secreted proteins, lipids, and extracellular DNA. This structural matrix poses a major challenge against common treatment options due to its extensive antibiotic-resistant properties. Because biofilms are so recalcitrant to antibiotics, they pose a unique challenge to patients in a nosocomial setting, mainly linked to lower respiratory, urinary tract, and surgical wound infections as well as the medical devices used during treatment. Another unique property of biofilm is its ability to adhere to both biological and man-made surfaces, allowing growth on human tissues and organs, hospital tools, and medical devices, etc. Based on prior understanding of bacteriophage structure, mechanisms, and its effects on bacteria eradication, leading research has been conducted on the effects of phages and its individual proteins on biofilm and its role in overall biofilm removal while also revealing the obstacles this form of treatment currently have. The expansion in the phage host-species range is one that urges for improvement and is the focus for future studies. This review aims to demonstrate the advantages and challenges of bacteriophage and its components on biofilm removal, as well as potential usage of phage cocktail, combination therapy, and genetically modified phages in a clinical setting.
Keywords: bacteriophages; biofilms; depolymerase; endolysin; phage therapy.
Copyright © 2022 Chang, Yu, Guo, Guo, Guo, Li and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

