Linear Six-Carbon Sugar Alcohols Induce Lysis of Microcystis aeruginosa NIES-298 Cells
- PMID: 35495711
- PMCID: PMC9039742
- DOI: 10.3389/fmicb.2022.834370
Linear Six-Carbon Sugar Alcohols Induce Lysis of Microcystis aeruginosa NIES-298 Cells
Abstract
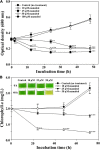
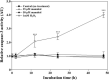
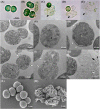
Cyanobacterial blooms are a global concern due to their adverse effects on water quality and human health. Therefore, we examined the effects of various compounds on Microcystis aeruginosa growth. We found that Microcystis aeruginosa NIES-298 cells were lysed rapidly by linear six-carbon sugar alcohols including mannitol, galactitol, iditol, fucitol, and sorbitol, but not by other sugar alcohols. Microscopic observations revealed that mannitol treatment induced crumpled inner membrane, an increase in periplasmic space, uneven cell surface with outer membrane vesicles, disruption of membrane structures, release of intracellular matter including chlorophylls, and eventual cell lysis in strain NIES-298, which differed from the previously proposed cell death modes. Mannitol metabolism, antioxidant-mediated protection of mannitol-induced cell lysis by, and caspase-3 induction in strain NIES-298 were not observed, suggesting that mannitol may not cause organic matter accumulation, oxidative stress, and programmed cell death in M. aeruginosa. No significant transcriptional expression was induced in strain NIES-298 by mannitol treatment, indicating that cell lysis is not induced through transcriptional responses. Mannitol-induced cell lysis may be specific to strain NIES-298 and target a specific component of strain NIES-298. This study will provide a basis for controlling M. aeruginosa growth specifically by non-toxic substances.
Keywords: cell lysis; cyanobacterial bloom; mannitol; outer membrane vesicle; six-carbon sugar alcohol.
Copyright © 2022 Jung, Seo, Jeong, Baek, Park and Jeon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Al-Tebrineh J., Merrick C., Ryan D., Humpage A., Bowling L., Neilan B. A. (2012). Community composition, toxigenicity, and environmental conditions during a cyanobacterial bloom occurring along 1,100 kilometers of the Murray River. Appl. Environ. Microbiol. 78 263–272. 10.1128/AEM.05587-11 - DOI - PMC - PubMed
-
- Berman-Frank I., Bidle K. D., Haramaty L., Falkowski P. G. (2004). The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol. Oceanogr. 49 997–1005. 10.4319/lo.2004.49.4.0997 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

