Amine decorated polystyrene nanobeads incorporating π-conjugated OPV chromophore for picric acid sensing in water
- PMID: 35496004
- PMCID: PMC9049647
- DOI: 10.1039/c9ra09852f
Amine decorated polystyrene nanobeads incorporating π-conjugated OPV chromophore for picric acid sensing in water
Abstract
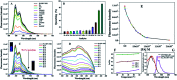
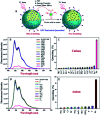
A solution as well as solid state based sensor has been developed for selective detection of picric acid (PA) in water. Oligo (p-phenylenevinylene) (OPV) incorporated polystyrene nanobeads (PS-OPV-NH2) having an average size of 180 nm have been synthesized through miniemulsion polymerization. Amine (-NH2) functionalization was performed on the nanobead surface to enhance the efficiency of the sensor among a library of other nitro-organics and library of cations and anions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Toal S. J. Trogler W. C. J. Mater. Chem. 2006;16:2871–2883. doi: 10.1039/B517953J. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

