Responsive Neurostimulation Targeting the Anterior, Centromedian and Pulvinar Thalamic Nuclei and the Detection of Electrographic Seizures in Pediatric and Young Adult Patients
- PMID: 35496067
- PMCID: PMC9039390
- DOI: 10.3389/fnhum.2022.876204
Responsive Neurostimulation Targeting the Anterior, Centromedian and Pulvinar Thalamic Nuclei and the Detection of Electrographic Seizures in Pediatric and Young Adult Patients
Abstract
Background: Responsive neurostimulation (RNS System) has been utilized as a treatment for intractable epilepsy. The RNS System delivers stimulation in response to detected abnormal activity, via leads covering the seizure foci, in response to detections of predefined epileptiform activity with the goal of decreasing seizure frequency and severity. While thalamic leads are often implanted in combination with cortical strip leads, implantation and stimulation with bilateral thalamic leads alone is less common, and the ability to detect electrographic seizures using RNS System thalamic leads is uncertain.
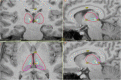
Objective: The present study retrospectively evaluated fourteen patients with RNS System depth leads implanted in the thalamus, with or without concomitant implantation of cortical strip leads, to determine the ability to detect electrographic seizures in the thalamus. Detailed patient presentations and lead trajectories were reviewed alongside electroencephalographic (ECoG) analyses.
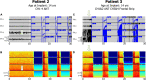
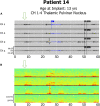
Results: Anterior nucleus thalamic (ANT) leads, whether bilateral or unilateral and combined with a cortical strip lead, successfully detected and terminated epileptiform activity, as demonstrated by Cases 2 and 3. Similarly, bilateral centromedian thalamic (CMT) leads or a combination of one centromedian thalamic alongside a cortical strip lead also demonstrated the ability to detect electrographic seizures as seen in Cases 6 and 9. Bilateral pulvinar leads likewise produced reliable seizure detection in Patient 14. Detections of electrographic seizures in thalamic nuclei did not appear to be affected by whether the patient was pediatric or adult at the time of RNS System implantation. Sole thalamic leads paralleled the combination of thalamic and cortical strip leads in terms of preventing the propagation of electrographic seizures.
Conclusion: Thalamic nuclei present a promising target for detection and stimulation via the RNS System for seizures with multifocal or generalized onsets. These areas provide a modifiable, reversible therapeutic option for patients who are not candidates for surgical resection or ablation.
Keywords: RNS; anterior thalamic nucleus; centromedian thalamic nucleus; epilepsy surgery; intractable epilepsy; pulvinar; responsive neurostimulation; thalamic stimulation.
Copyright © 2022 Beaudreault, Muh, Naftchi, Spirollari, Das, Vazquez, Sukul, Overby, Tobias, McGoldrick and Wolf.
Conflict of interest statement
SW and PM reports honoraria from LivaNova, Eisai, UCB, Sunovion, Greenwich Pharmaceuticals, and participation as an investigator in clinical trials for Zogenix, GW Pharma, NeuroPace, Neurelis, UCB, Eisai. CM reports speaker fees from LivaNova, and participation as an investigator in clinical trial for NeuroPace. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor GW declared a past co-authorship with the author(s) SW and PM.
Figures



References
LinkOut - more resources
Full Text Sources

