An Open-Label, Randomized, Prospective, Comparative, Three-Arm Clinical Trial to Evaluate the Safety and Effectiveness of Apremilast with Three Different Titration Methods in Patients with Chronic Plaque Psoriasis in India
- PMID: 35496380
- PMCID: PMC9041601
- DOI: 10.2147/PTT.S357184
An Open-Label, Randomized, Prospective, Comparative, Three-Arm Clinical Trial to Evaluate the Safety and Effectiveness of Apremilast with Three Different Titration Methods in Patients with Chronic Plaque Psoriasis in India
Abstract
Purpose: To minimize adverse effects (AEs), apremilast is recommended to titrate at the initiation of therapy. But still, many patients experience AEs, resulting in discontinuation of therapy. As a result, many dermatologists have adapted to further titrate apremilast in different ways. The present study was planned to evaluate the safety and effectiveness of apremilast in different dose titration methods as initiation therapy in the treatment of plaque psoriasis.
Patients and methods: In this open-label, randomized, prospective, comparative, three-arm, single center study, 128 plaque psoriasis patients were included. Patients were randomized into three groups. Group I received standard titration for the first 6 days; Group II received all tablets in a starter pack as once a day (OD) total for 13 days; and Group III received two starter packs as 8 tablets each of apremilast 10 mg and 20 mg as OD and 10 tablets of 30 mg as OD, in total for 26 days. All groups received apremilast 30 mg as twice a day after initial titration. The total duration of apremilast therapy in all groups was 16 weeks.
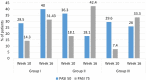
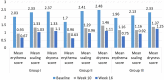
Results: In safety assessment, AEs were reported in 50%, 41.3% and 25% in Groups I, II and III, respectively (p <0.05) with nausea being the most common AE. In Group I, 10.53% of patients discontinued apremilast whereas 6.52% and 2.27% discontinued in Groups II and III respectively. Maximum number of AEs were seen in Group I in first week only (74.19%) compared with other groups. At week 16, on the Psoriasis Area and Severity Index, PASI 75 was achieved in 31.43%, 42.4% and 33.3% of patients in Groups I, II and III, respectively with no statistical difference between any groups.
Conclusion: It can be concluded that slower titration is a useful strategy for minimizing AEs while at the same time maintaining effectiveness of apremilast.
Keywords: adverse events; apremilast; effectiveness; titration.
© 2022 Viswanath et al.
Conflict of interest statement
Dr Dhiraj Dhoot, Dr Namrata Mahadkar and Dr Hanmant Barkate are employees of Glenmark Pharmaceuticals Ltd. The other authors report no conflicts of interest in this work.
Figures
References
-
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a Phase III, randomized, controlled trial (efficacy and safety trial evaluating the effects of apremilast in psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73(1):37–49. doi:10.1016/j.jaad.2015.03.049 - DOI - PubMed
-
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–1399. doi:10.1111/bjd.14164 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials