Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: synthesis and evaluation of cytotoxic effects
- PMID: 35496404
- PMCID: PMC9043769
- DOI: 10.1039/d1ra07613b
Novel alkylaminoethyl derivatives of androstane 3-oximes as anticancer candidates: synthesis and evaluation of cytotoxic effects
Abstract
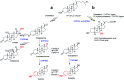
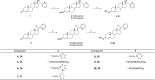
Steroid anticancer drugs are the focus of numerous scientific research efforts. Due to their high cytotoxic effects against tumor cells, some natural or synthetic steroid compounds seem to be promising for the treatment of different classes of cancer. In the present study, fourteen novel O-alkylated oxyimino androst-4-ene derivatives were synthesized from isomerically pure 3E-oximes, using different alkylaminoethyl chlorides. Their in vitro cytotoxic activity was evaluated against eight human cancer cell lines, as well as against normal fetal lung (MRC-5) and human foreskin (BJ) fibroblasts, to test the efficiency and selectivity of the compounds. Most derivatives displayed strong activity against malignant melanoma (G-361), lung adenocarcinoma (A549) and colon adenocarcinoma (HT-29) cell lines. Angiogenesis was assessed in vitro using migration scratch and tube formation assays on HUVEC cells, where partial inhibition of endothelial cell migration was observed for the 17α-(pyridin-2-yl)methyl 2-(morpholin-4-yl)ethyl derivative. Among the compounds that most impaired the growth of lung cancer A549 cells, the (17E)-(pyridin-2-yl)methylidene derivative bearing a 2-(pyrrolidin-1-yl)ethyl substituent induced significant apoptosis in these cells. In combination with low cytotoxicity toward normal MRC-5 cells, this molecule stands out as a good candidate for further anticancer studies. In addition, in vitro investigations against cytochrome P450 enzymes revealed that certain compounds can bind selectively in the active sites of human steroid hydroxylases CYP7, CYP17A1, CYP19A1 or CYP21A2, which could be important for the development of novel activity modulators of these enzymes and identification of possible side effects.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



Similar articles
-
Anticancer potential of new steroidal thiazolidin-4-one derivatives. Mechanisms of cytotoxic action and effects on angiogenesis in vitro.J Steroid Biochem Mol Biol. 2017 Nov;174:72-85. doi: 10.1016/j.jsbmb.2017.07.031. Epub 2017 Jul 26. J Steroid Biochem Mol Biol. 2017. PMID: 28756292
-
Novel tetrahydroacridine derivatives with iodobenzoic moieties induce G0/G1 cell cycle arrest and apoptosis in A549 non-small lung cancer and HT-29 colorectal cancer cells.Mol Cell Biochem. 2019 Oct;460(1-2):123-150. doi: 10.1007/s11010-019-03576-x. Epub 2019 Jul 16. Mol Cell Biochem. 2019. PMID: 31313023 Free PMC article.
-
Androstane derivatives induce apoptotic death in MDA-MB-231 breast cancer cells.Bioorg Med Chem. 2015 Nov 15;23(22):7189-98. doi: 10.1016/j.bmc.2015.10.015. Epub 2015 Oct 19. Bioorg Med Chem. 2015. PMID: 26494582
-
Synthesis and anticancer potential of novel 5,6-oxygenated and/or halogenated steroidal d-homo lactones.Bioorg Med Chem. 2021 Jan 15;30:115935. doi: 10.1016/j.bmc.2020.115935. Epub 2020 Dec 9. Bioorg Med Chem. 2021. PMID: 33340938
-
Steroidal Oximes: Useful Compounds with Antitumor Activities.Curr Med Chem. 2018 Feb 21;25(6):660-686. doi: 10.2174/0929867324666171003115400. Curr Med Chem. 2018. PMID: 28971759 Review.
Cited by
-
The Structural Diversity and Biological Activity of Steroid Oximes.Molecules. 2023 Feb 10;28(4):1690. doi: 10.3390/molecules28041690. Molecules. 2023. PMID: 36838678 Free PMC article. Review.
-
A Review of Biologically Active Oxime Ethers.Molecules. 2023 Jun 28;28(13):5041. doi: 10.3390/molecules28135041. Molecules. 2023. PMID: 37446703 Free PMC article. Review.
-
The Fundamental Role of Oxime and Oxime Ether Moieties in Improving the Physicochemical and Anticancer Properties of Structurally Diverse Scaffolds.Int J Mol Sci. 2023 Nov 28;24(23):16854. doi: 10.3390/ijms242316854. Int J Mol Sci. 2023. PMID: 38069175 Free PMC article. Review.
References
-
- GLOBOCAN 2020. New Global Cancer Data, International Agency for Research on Cancer (IARC), Geneva, Switzerland, 2020, https://www.uicc.org/news/globocan-2020-new-global-cancer-data, accessed June 2021
-
- Fenton R. G. and Longo D. L., in Harrison's Principles of Internal Medicine, ed. A. S. Fauci, E. Braunwald, K. J. Isselbachner, J. D. Wilson, J. B. Martin, D. L. Kasper, S. L. Hauser and D. L Longo, McGraw Hill, New York, 1998, pp. 505–511
LinkOut - more resources
Full Text Sources

