Recent advances in the transition-metal-free synthesis of quinoxalines
- PMID: 35496411
- PMCID: PMC9043781
- DOI: 10.1039/d1ra06942j
Recent advances in the transition-metal-free synthesis of quinoxalines
Abstract
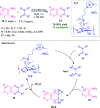
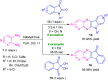
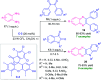
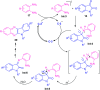
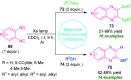
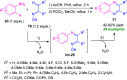
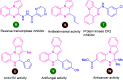
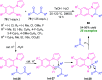
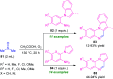
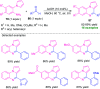
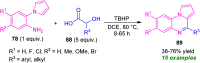
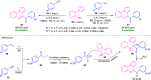
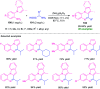
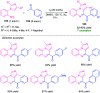
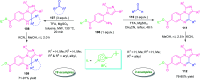
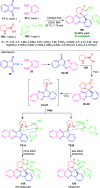
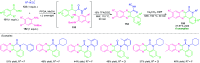
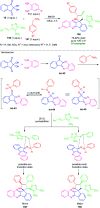
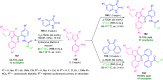
Quinoxalines, also known as benzo[a]pyrazines, constitute an important class of nitrogen-containing heterocyclic compounds as a result of their widespread prevalence in natural products, biologically active synthetic drug candidates, and optoelectronic materials. Owing to their importance and chemists' ever-increasing imagination of new transformations of these products, tremendous efforts have been dedicated to finding more efficient approaches toward the synthesis of quinoxaline rings. The last decades have witnessed a marvellous outburst in modifying organic synthetic methods to create them sustainable for the betterment of our environment. The exploitation of transition-metal-free catalysis in organic synthesis leads to a new frontier to access biologically active heterocycles and provides an alternative method from the perspective of green and sustainable chemistry. Despite notable developments achieved in transition-metal catalyzed synthesis, the high cost involved in the preparation of the catalyst, toxicity, and difficulty in removing it from the final products constitute disadvantageous effects on the atom economy and eco-friendly nature of the transformation. In this review article, we have summarized the recent progress achieved in the synthesis of quinoxalines under transition-metal-free conditions and cover the reports from 2015 to date. This aspect is presented alongside the mechanistic rationalization and limitations of the reaction methodologies. The scopes of future developments are also highlighted.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
“There are no conflicts to declare”.
Figures

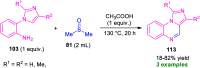


Similar articles
-
Recent Advances in the Transition-Metal-Free Synthesis of Quinazolines.Molecules. 2023 Apr 4;28(7):3227. doi: 10.3390/molecules28073227. Molecules. 2023. PMID: 37049989 Free PMC article. Review.
-
Reusable nano-catalyzed green protocols for the synthesis of quinoxalines: an overview.RSC Adv. 2023 Jul 7;13(29):20373-20406. doi: 10.1039/d3ra03646d. eCollection 2023 Jun 29. RSC Adv. 2023. PMID: 37425629 Free PMC article. Review.
-
Alkynoates as Versatile and Powerful Chemical Tools for the Rapid Assembly of Diverse Heterocycles under Transition-Metal Catalysis: Recent Developments and Challenges.Top Curr Chem (Cham). 2021 Jan 5;379(1):3. doi: 10.1007/s41061-020-00316-4. Top Curr Chem (Cham). 2021. PMID: 33398642 Review.
-
Transition-metal-catalyzed synthesis of quinazolines: A review.Front Chem. 2023 Mar 16;11:1140562. doi: 10.3389/fchem.2023.1140562. eCollection 2023. Front Chem. 2023. PMID: 37007059 Free PMC article. Review.
-
Copper-Catalyzed Oxidative Carbon-Carbon and/or Carbon-Heteroatom Bond Formation with O2 or Internal Oxidants.Acc Chem Res. 2018 May 15;51(5):1092-1105. doi: 10.1021/acs.accounts.7b00611. Epub 2018 Apr 12. Acc Chem Res. 2018. PMID: 29648789
Cited by
-
Recent advances and prospects in the organocatalytic synthesis of quinazolinones.Front Chem. 2022 Sep 14;10:991026. doi: 10.3389/fchem.2022.991026. eCollection 2022. Front Chem. 2022. PMID: 36186594 Free PMC article. Review.
-
Catalytic acidic deep eutectic mixture for efficient and promising synthesis of quinazolinone and quinoxaline derivatives.RSC Adv. 2025 Jul 21;15(32):25971-25984. doi: 10.1039/d5ra03346b. eCollection 2025 Jul 21. RSC Adv. 2025. PMID: 40697466 Free PMC article.
-
Ultrasound-assisted transition-metal-free catalysis: a sustainable route towards the synthesis of bioactive heterocycles.RSC Adv. 2022 May 11;12(22):14022-14051. doi: 10.1039/d2ra02063g. eCollection 2022 May 5. RSC Adv. 2022. PMID: 35558846 Free PMC article. Review.
-
Brønsted acid catalyzed mechanochemical domino multicomponent reactions by employing liquid assisted grindstone chemistry.Sci Rep. 2023 Jan 25;13(1):1386. doi: 10.1038/s41598-023-27948-y. Sci Rep. 2023. PMID: 36697475 Free PMC article.
-
Highly enantioselective synthesis of both enantiomers of tetrahydroquinoxaline derivatives via Ir-catalyzed asymmetric hydrogenation.Chem Sci. 2024 Aug 23;15(37):15243-54. doi: 10.1039/d4sc04222k. Online ahead of print. Chem Sci. 2024. PMID: 39246375 Free PMC article.
References
-
- Joule J. A. Adv. Heterocycl. Chem. 2016;119:81–106. doi: 10.1016/bs.aihch.2015.10.005. - DOI
- Walsh C. T. Tetrahedron Lett. 2015;56:3075–3081. doi: 10.1016/j.tetlet.2014.11.046. - DOI
- Kerru N. Gummidi L. Maddila S. Gangu K. K. Jonnalagadda S. B. Molecules. 2020;25:1909. doi: 10.3390/molecules25081909. - DOI - PMC - PubMed
- Chugh A. Kumar A. Verma A. Kumar S. Kumar P. Med. Chem. Res. 2020;29:1723–1750. doi: 10.1007/s00044-020-02604-6. - DOI
- Lipunova G. N. Nosova E. V. Charushin V. N. Chupakhin O. N. Curr. Org. Synth. 2018;15:793–814. doi: 10.2174/1570179415666180622123434. - DOI
- Chen D. Su S. J. Cao Y. J. Mater. Chem. C. 2014;2:9565–9578. doi: 10.1039/C4TC01941E. - DOI
-
- Jadhavar P. S., Kumar D., Purohit P., Pipaliya B. V., Kumar A., Bhagat S. and Chakraborti A. K., Sustainable approaches towards the synthesis of quinoxalines, in Green Chemistry: Synthesis of Bioactive Heterocycles, Springer, New Delhi, 2014, pp. 37–67
- Pereira J. A. Pessoa A. M. Cordeiro M. N. D. Fernandes R. Prudêncio C. Noronha J. P. Vieira M. Eur. J. Med. Chem. 2015;97:664–672. doi: 10.1016/j.ejmech.2014.06.058. - DOI - PubMed
- Patidar A. K. Jeyakandan M. Mobiya A. K. Selvam G. Int. J. PharmTech Res. 2011;3:386–392.
- Kaushal T. Srivastava G. Sharma A. Negi A. S. Bioorg. Med. Chem. 2019;27:16–35. doi: 10.1016/j.bmc.2018.11.021. - DOI - PubMed
- Irfan A. Ahmad S. Hussain S. Batool F. Riaz H. Zafar R. Mojzych M. Appl. Sci. 2021;11:5702. doi: 10.3390/app11125702. - DOI
-
- Martin D. G. Mizsak S. A. Biles C. Stewart J. C. Baczynskyj L. Meulman P. A. J. Antibiot. 1975;28:332–336. doi: 10.7164/antibiotics.28.332. - DOI - PubMed
- Watanabe K. Biosci., Biotechnol., Biochem. 2008;72:2491–2506. doi: 10.1271/bbb.80323. - DOI - PubMed
- Waring M. J., Echinomycin and related quinoxaline antibiotics, in Molecular Aspects of Anticancer Drug-DNA Interactions, Palgrave, London, 1993, pp. 213–242
- Matsuura S. J. Antibiot., Ser. A. 1965;18:43–46. - PubMed
- Hayakawa Y. Sone R. Aoki H. Kimata S. J. Antibiot. 2018;71:898–901. doi: 10.1038/s41429-018-0083-6. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

