Ag@BiOCl super-hydrophobic nanostructure for enhancing SERS detection sensitivity
- PMID: 35496623
- PMCID: PMC9050507
- DOI: 10.1039/d0ra01226b
Ag@BiOCl super-hydrophobic nanostructure for enhancing SERS detection sensitivity
Abstract
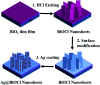
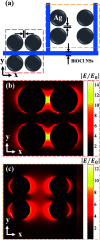
Surface-enhanced Raman scattering (SERS) has received widespread attention in the rapid detection of trace substances. The super-hydrophobic surface of structures has a significant impact on improving SERS performance. Usually a low concentration of objective molecules is randomly distributed in a large area on a non-hydrophobic SERS substrate, resulting in the Raman signals of the molecules not being easily detected. As a solution, a super-hydrophobic surface can gather a large number of probe molecules around the plasmon hot spots to effectively improve Raman SERS detection sensitivity. In this work, a chloride super-hydrophobic surface is fabricated, for the first time, by a simple and low-cost method of combining surface hydrophobic structures with surface modification. The dispersed and uniform hierarchical Ag@BiOCl nanosheet (Ag@BiOCl NSs) substrate has a higher surface-to-volume ratio and rich nano-gap. Such a chip with a high static contact angle of 157.4° exhibits a Raman signal detection limit of R6G dyes up to 10-9 M and an enhancement factor up to 107. This SERS chip with a super-hydrophobic surface offers great potential in practical applications owing to its simple fabricating process, low cost, large area, and high sensitivity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Evaporation-induced self-assembly of silver nanospheres and gold nanorods on a super-hydrophobic substrate for SERS applications.Nanotechnology. 2021 Mar 26;32(13):135601. doi: 10.1088/1361-6528/abd1aa. Nanotechnology. 2021. PMID: 33291094
-
Fabrication of Ag based Surface Enhanced Raman Scattering substrates with periodic mask arrays by electron beam deposition.Anal Chim Acta. 2025 Feb 22;1340:343666. doi: 10.1016/j.aca.2025.343666. Epub 2025 Jan 13. Anal Chim Acta. 2025. PMID: 39863314
-
Ag gyrus-nanostructure supported on graphene/Au film with nanometer gap for ideal surface enhanced Raman scattering.Opt Express. 2017 Aug 21;25(17):20631-20641. doi: 10.1364/OE.25.020631. Opt Express. 2017. PMID: 29041742
-
Analysis on superhydrophobic silver decorated copper Oxide nanostructured thin films for SERS studies.J Colloid Interface Sci. 2016 Sep 1;477:209-19. doi: 10.1016/j.jcis.2016.05.051. Epub 2016 May 25. J Colloid Interface Sci. 2016. PMID: 27294970
-
Super-Resolution Surface-Enhanced Raman Scattering: Perspectives on the Past, Present, and Future.ACS Nano. 2024 Oct 15;18(41):27824-27832. doi: 10.1021/acsnano.4c10655. Epub 2024 Oct 1. ACS Nano. 2024. PMID: 39353138 Review.
Cited by
-
HPTLC screening of saccharin in beverages by densitometry quantification and SERS confirmation.RSC Adv. 2022 Mar 16;12(14):8317-8322. doi: 10.1039/d1ra09416e. eCollection 2022 Mar 15. RSC Adv. 2022. PMID: 35424832 Free PMC article.
-
Arrayed nanopore silver thin films for surface-enhanced Raman scattering.RSC Adv. 2020 Jun 23;10(40):23908-23915. doi: 10.1039/d0ra03803b. eCollection 2020 Jun 19. RSC Adv. 2020. PMID: 35517352 Free PMC article.
References
-
- Tripp R. A. Dluhy R. A. Zhao Y. P. Nano Today. 2008;3:31–37. doi: 10.1016/S1748-0132(08)70042-2. - DOI
-
- Vo-Dinh T. Yan F. Wabuyele M. B. J. Raman Spectrosc. 2005;36:640–647. doi: 10.1002/jrs.1348. - DOI
-
- Alvarez-Puebla R. A. Liz-Marzan L. M. Energy Environ. Sci. 2010;3:1011–1017. doi: 10.1039/C002437F. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

