Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay
- PMID: 35496625
- PMCID: PMC9050516
- DOI: 10.1039/d0ra00062k
Microfluidic paper device for rapid detection of aflatoxin B1 using an aptamer based colorimetric assay
Abstract
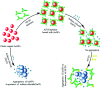
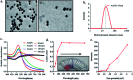
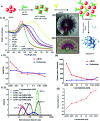
Contamination of milk by mycotoxins is a serious problem worldwide. Herein, we focused on the detection of aflatoxin B1 (AflB1) using a paper microfluidic device fabricated with specific aptamers as biorecognition elements. The fabrication process resulted in the generation of a leak proof microfluidic device where two zones were designed: control and test. Detection is achieved by color change when aflatoxin reacts with an aptamer followed by salt induced aggregation of gold nanoparticles. Specific aptamers for aflatoxin B1 were immobilized successfully onto the surface of gold nanoparticles. Biophysical characterization of the conjugated AuNPs-aptamer was done by UV-vis spectroscopy, DLS (dynamic light scattering), TEM (transmission electron microscopy). Under optimal conditions, the microfluidic device showed an excellent response for aflatoxin B1 detection in the range of 1 pM to 1 μM with a detection limit of up to 10 nM in spiked samples. Disadvantages associated with conventional techniques of ELISA were overcome by this one step detection technique with low operation cost, simple instrumentation, and user-friendly format with no interference due to chromatographic separation. The developed microfluidic paper-based analytical device (μPAD) can provide a tool for on-site detection of food toxins in less than a minute which is the main requirement for both qualitative and quantitative analysis in food safety and environmental monitoring.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict of interest among all authors of the papers.
Figures


Similar articles
-
Fabrication of microfluidic device for Aflatoxin M1 detection in milk samples with specific aptamers.Sci Rep. 2020 Mar 13;10(1):4627. doi: 10.1038/s41598-020-60926-2. Sci Rep. 2020. PMID: 32170077 Free PMC article.
-
Molecular Diagnostic of Ochratoxin A With Specific Aptamers in Corn and Groundnut via Fabrication of a Microfluidic Device.Front Nutr. 2022 Mar 24;9:851787. doi: 10.3389/fnut.2022.851787. eCollection 2022. Front Nutr. 2022. PMID: 35399674 Free PMC article.
-
Colorimetric detection of the potent carcinogen aflatoxin B1 based on the aggregation of L-lysine-functionalized gold nanoparticles in the presence of copper ions.Front Nutr. 2024 Jun 6;11:1425638. doi: 10.3389/fnut.2024.1425638. eCollection 2024. Front Nutr. 2024. PMID: 38903616 Free PMC article.
-
AFB1 colorimetric aptamer sensor for the detection of AFB1 in ten different kinds of miscellaneous beans based on gold nanoparticles and smartphone imaging.Food Chem. 2023 Sep 30;421:136205. doi: 10.1016/j.foodchem.2023.136205. Epub 2023 Apr 19. Food Chem. 2023. PMID: 37094407
-
Aptamer-based Colorimetric and Chemiluminescence Detection of Aflatoxin B1 in Foods Samples.Acta Chim Slov. 2015;62(3):721-8. doi: 10.17344/acsi.2015.1358. Acta Chim Slov. 2015. PMID: 26466094
Cited by
-
Advances in Colorimetric Strategies for Mycotoxins Detection: Toward Rapid Industrial Monitoring.Toxins (Basel). 2020 Dec 24;13(1):13. doi: 10.3390/toxins13010013. Toxins (Basel). 2020. PMID: 33374434 Free PMC article. Review.
-
Gold Tablets: Gold Nanoparticles Encapsulated into Dextran Tablets and Their pH-Responsive Behavior as an Easy-to-Use Platform for Multipurpose Applications.ACS Omega. 2022 Mar 22;7(13):11177-11189. doi: 10.1021/acsomega.1c07393. eCollection 2022 Apr 5. ACS Omega. 2022. PMID: 35415343 Free PMC article.
-
Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies.Toxins (Basel). 2022 Oct 6;14(10):687. doi: 10.3390/toxins14100687. Toxins (Basel). 2022. PMID: 36287956 Free PMC article. Review.
-
Increasing the packing density of assays in paper-based microfluidic devices.Biomicrofluidics. 2021 Feb 4;15(1):011502. doi: 10.1063/5.0042816. eCollection 2021 Jan. Biomicrofluidics. 2021. PMID: 33569089 Free PMC article. Review.
-
Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review.Toxins (Basel). 2022 Apr 27;14(5):307. doi: 10.3390/toxins14050307. Toxins (Basel). 2022. PMID: 35622554 Free PMC article. Review.
References
-
- International Agency for Research on Cancer (IARC), Evaluation of carcinogenic risks in humans, Lyon (France), 2002, vol. 82, pp. 171–274
-
- Leitner A. Zöllner P. Paolillo A. Stroka J. Papadopoulou-Bouraoui A. Jaborek S. Anklam E. Lindner W. Anal. Chim. Acta. 2002;453:33–41. doi: 10.1016/S0003-2670(01)01483-0. - DOI
-
- Wacoo A. Wendiro D. Vuzi P. Hawumba J. J. Appl. Chem. 2014;706291:1–15.
Grants and funding
LinkOut - more resources
Full Text Sources

