Barium promoted Ni/Sm2O3 catalysts for enhanced CO2 methanation
- PMID: 35496871
- PMCID: PMC9041535
- DOI: 10.1039/d1ra04115k
Barium promoted Ni/Sm2O3 catalysts for enhanced CO2 methanation
Abstract
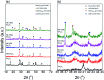
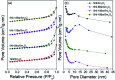
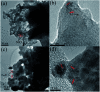
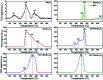
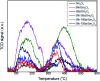
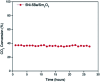
Low temperature CO2 methanation is a favorable pathway to achieve high selectivity to methane while increasing the stability of the catalysts. A Ba promoted Ni/Sm2O3 catalyst was investigated for CO2 methanation at atmospheric pressure with the temperature ranging from 200-450 °C. 5Ni-5Ba/Sm2O3 showed significant enhancement of CO2 conversion particularly at temperatures ≤ 300 °C compared to Ni/Sm2O3. Incorporation of Ba into 5Ni/Sm2O3 improved the basicity of the catalysts and transformed the morphology of Sm2O3 from random structure into uniform groundnut shape nanoparticles. The uniformity of Sm2O3 created interparticle porosity that may be responsible for efficient heat transfer during a long catalytic reaction. Ba is also postulated to catalyze oxygen vacancy formation on Sm2O3 under a reducing environment presumably via isomorphic substitution. The disappearance of a high temperature (∼600 °C) reduction peak in H2-TPR analysis revealed the reducibility of NiO following impregnation with Ba. However, further increasing the Ba loading to 15% formed BaNiO3-BaNiO2.36 phases which consequently reduced the activity of the Ni-Ba/Sm2O3 catalyst at low temperature. Ni was suggested to segregate from BaNiO3-BaNiO2.36 at high temperature thus exhibiting comparable activity with Ni/Sm2O3 at 450 °C.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Cuéllar-Franca R. M. Azapagic A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J CO2 Util. 2015;9:82–102. doi: 10.1016/j.jcou.2014.12.001. - DOI
-
- Martin N. M. Velin P. Skoglundh M. Bauer M. Carlsson P. A. Catalytic hydrogenation of CO2 to methane over supported Pd, Rh and Ni catalysts. Catal. Sci. Technol. 2017;7(5):1086–1094. doi: 10.1039/C6CY02536F. doi: 10.1039/C6CY02536F. - DOI
-
- Ashok J. Pati S. Hongmanorom P. Tianxi Z. Junmei C. Kawi S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today. 2020;356:471–489. doi: 10.1016/j.cattod.2020.07.023. doi: 10.1016/j.cattod.2020.07.023. - DOI
LinkOut - more resources
Full Text Sources

