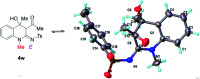A simple and efficient copper-catalyzed three-component reaction to synthesize (Z)-1,2-dihydro-2-iminoquinolines
- PMID: 35496874
- PMCID: PMC9041411
- DOI: 10.1039/d1ra06330h
A simple and efficient copper-catalyzed three-component reaction to synthesize (Z)-1,2-dihydro-2-iminoquinolines
Abstract
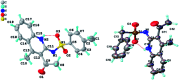
A operationally simple synthesis of (Z)-1,2-dihydro-2-iminoquinolines that proceeds under mild conditions is achieved by copper-catalyzed reaction of 1-(2-aminophenyl)ethan-1-ones, sulfonyl azides and terminal ynones. In particular, the reaction goes through a base-free CuAAC/ring-opening process to obtain the Z-configured products due to hydrogen bonding.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Chen L. Deng Z. Zhao C. ACS Chem. Biol. 2021;16:559. doi: 10.1021/acschembio.1c00052. - DOI - PubMed
- Chambers G. E. Sayan A. E. Brown R. C. D. Nat. Prod. Rep. 2021 doi: 10.1039/D0NP00096E. - DOI - PubMed
- Bhambhani S. Kondhare K. R. Giri A. P. Molecules. 2021;26:3374. doi: 10.3390/molecules26113374. - DOI - PMC - PubMed
- Wang A. Li P. Han P. Gu G. Shan T. Lai D. Zhou L. Nat. Prod. Res. 2021;35:272. doi: 10.1080/14786419.2019.1627354. - DOI - PubMed
- Cinelli M. A. Jones A. D. Molecules. 2021;26:2629. doi: 10.3390/molecules26092629. - DOI - PMC - PubMed
- Asamizu S. Biosci., Biotechnol., Biochem. 2017;81:871. doi: 10.1080/09168451.2017.1281726. - DOI - PubMed
- Chattopadhyay A. K. Hanessian S. Chem. Rev. 2017;117:4104. doi: 10.1021/acs.chemrev.6b00412. - DOI - PubMed
- Hu J. F. Fan H. Xiong J. Wu S. B. Chem. Rev. 2011;111:5465. doi: 10.1021/cr100435g. - DOI - PubMed
- Padwa A. Chem. Soc. Rev. 2009;38:3072. doi: 10.1039/B816701J. - DOI - PubMed
- Fan H. Peng J. Hamann M. T. Hu J. F. Chem. Rev. 2008;108:264. doi: 10.1021/cr078199m. - DOI - PMC - PubMed
-
- Hügel H. M. de Silva N. H. Siddiqui A. Blanch E. Lingham A. Bioorg. Med. Chem. 2021;43:116270. doi: 10.1016/j.bmc.2021.116270. - DOI - PubMed
- Huang D. N. Wu F. F. Zhang A. H. Sun H. Wang X. J. Pharmacol. Res. 2021;169:105667. doi: 10.1016/j.phrs.2021.105667. - DOI - PubMed
- Ghanbari-Movahed M. Kaceli T. Mondal A. Farzaei M. H. Bishayee A. Biomedicines. 2021;9:480. doi: 10.3390/biomedicines9050480. - DOI - PMC - PubMed
- Youssef F. S. Simal-Gandara J. Biomedicines. 2021;9:485. doi: 10.3390/biomedicines9050485. - DOI - PMC - PubMed
-
- Martorana A. La Monica G. Lauria A. Molecules. 2020;25:4279. doi: 10.3390/molecules25184279. - DOI - PMC - PubMed
- Nqoro X. Tobeka N. Aderibigbe B. A. Molecules. 2017;22:2268. doi: 10.3390/molecules22122268. - DOI - PMC - PubMed
- Vandekerckhove S. D'hooghe M. Bioorg. Med. Chem. 2015;23:5098. doi: 10.1016/j.bmc.2014.12.018. - DOI - PubMed
- Wadhwa P. Jain P. Rudrawar S. Jadhav H. R. A. Curr. Drug Discovery Technol. 2018;15:2. doi: 10.2174/1570163814666170531115452. - DOI - PubMed
LinkOut - more resources
Full Text Sources