Facile fabrication of bimetallic Fe-Mg MOF for the synthesis of xanthenes and removal of heavy metal ions
- PMID: 35497246
- PMCID: PMC9050136
- DOI: 10.1039/c9ra10300g
Facile fabrication of bimetallic Fe-Mg MOF for the synthesis of xanthenes and removal of heavy metal ions
Abstract
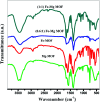
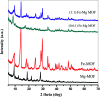
This work reported the preparation of Mg-MOF, Fe-MOF and Fe-Mg MOF by a solvothermal technique and their characterization with FT-IR, XRD, SEM, EDS, TEM and S BET analyses. The nanoparticle diameter ranged from 3.1 to 10.9 nm. The acidity of the MOFs was measured by nonaqueous potentiometric titration of n-butylamine. It was observed that the formation of a bimetallic MOF sharply increases the surface acidity and the catalytic activity. The catalytic results of the Fe-Mg MOF catalyzing the synthesis of 14-aryl-14-H-dibenzo[a,j]xanthenes in comparison with those of parent MOFs showed a higher yield of the desired product in a lower time and among various Fe : Mg, the (0.6 : 1) Fe-Mg MOF showed the highest catalytic activity and acidity. Even after the 4th run, the Fe-Mg MOF catalyst still maintained nearly the initial catalytic activity. The adsorption performance of Mg-MOF, Fe-MOF and Fe-Mg MOF was evaluated by batch experiments. The effect of contact time, the solution pH, the adsorbent dose and the initial concentration of the heavy metal ions was discussed. It was found that the capacity of the bimetallic Fe-Mg MOF for Pb(ii), Cu(ii) and Cd(ii) adsorption was higher than that of the Mg-MOF and Fe-MOF, the kinetic data followed the pseudo-second-order kinetic model and the isothermal data obeyed the Langmuir isotherm model. The mechanism of the removal of the heavy metal ions was discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





Similar articles
-
Synthesis of Highly Efficient and Recyclable Bimetallic Cox-Fe1-x-MOF for the Synthesis of Xanthan and Removal of Toxic Pb2+ and Cd2+ Ions.ACS Omega. 2023 Jul 15;8(29):26379-26390. doi: 10.1021/acsomega.3c02911. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521672 Free PMC article.
-
Facile immobilization of ethylenediamine tetramethylene-phosphonic acid into UiO-66 for toxic divalent heavy metal ions removal: An experimental and theoretical exploration.Sci Total Environ. 2022 Feb 1;806(Pt 3):150652. doi: 10.1016/j.scitotenv.2021.150652. Epub 2021 Oct 2. Sci Total Environ. 2022. PMID: 34610397
-
Cu-Co bimetallic organic framework as effective adsorbents for enhanced adsorptive removal of tetracycline antibiotics.Sci Rep. 2024 Jul 30;14(1):17607. doi: 10.1038/s41598-024-67986-8. Sci Rep. 2024. PMID: 39080297 Free PMC article.
-
Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions.J Hazard Mater. 2019 Apr 15;368:10-20. doi: 10.1016/j.jhazmat.2019.01.024. Epub 2019 Jan 11. J Hazard Mater. 2019. PMID: 30658159
-
Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites.RSC Adv. 2021 Mar 9;11(17):9977-9984. doi: 10.1039/d1ra01027a. eCollection 2021 Mar 5. RSC Adv. 2021. PMID: 35423486 Free PMC article.
Cited by
-
Introduction of a trinuclear manganese(iii) catalyst on the surface of magnetic cellulose as an eco-benign, efficient and reusable novel heterogeneous catalyst for the multi-component synthesis of new derivatives of xanthene.RSC Adv. 2021 Jan 21;11(8):4339-4355. doi: 10.1039/d0ra09420j. eCollection 2021 Jan 21. RSC Adv. 2021. Retraction in: RSC Adv. 2024 Jan 5;14(3):1671. doi: 10.1039/d3ra90130k. PMID: 35424405 Free PMC article. Retracted.
-
Oxidation of BINOLs by Hypervalent Iodine Reagents: Facile Synthesis of Xanthenes and Lactones.Chemistry. 2022 Apr 12;28(21):e202200181. doi: 10.1002/chem.202200181. Epub 2022 Mar 14. Chemistry. 2022. PMID: 35225370 Free PMC article.
-
NH2-MIL-88B (Fe α In1-α ) mixed-MOFs designed for enhancing photocatalytic Cr(vi) reduction and tetracycline elimination.RSC Adv. 2020 Oct 25;10(64):39080-39086. doi: 10.1039/d0ra07487j. eCollection 2020 Oct 21. RSC Adv. 2020. PMID: 35518441 Free PMC article.
-
Synthesis of Highly Efficient and Recyclable Bimetallic Cox-Fe1-x-MOF for the Synthesis of Xanthan and Removal of Toxic Pb2+ and Cd2+ Ions.ACS Omega. 2023 Jul 15;8(29):26379-26390. doi: 10.1021/acsomega.3c02911. eCollection 2023 Jul 25. ACS Omega. 2023. PMID: 37521672 Free PMC article.
-
Palladium supported on mixed-metal-organic framework (Co-Mn-MOF-74) for efficient catalytic oxidation of CO.RSC Adv. 2021 Jan 21;11(8):4318-4326. doi: 10.1039/d0ra09970h. eCollection 2021 Jan 21. RSC Adv. 2021. PMID: 35424392 Free PMC article.
References
-
- Zhang J. Xiong Z. Li C. Wu C. J. Mol. Liq. 2016;221:43–50. doi: 10.1016/j.molliq.2016.05.054. - DOI
-
- Ahmed I. Jhung S. H. Mater. Today. 2014;17:136–146. doi: 10.1016/j.mattod.2014.03.002. - DOI
-
- J-Alcaniz J. Gielisse R. Lago A. B. R-Fernandez E. V. S-Crespo P. Devic T. Guillou N. Serre C. Kapteijn F. Gascon J. Catal. Sci. Technol. 2013;3:2311–2318. doi: 10.1039/C3CY00272A. - DOI
LinkOut - more resources
Full Text Sources

