Chemosensors based on N-heterocyclic dyes: advances in sensing highly toxic ions such as CN- and Hg2
- PMID: 35497277
- PMCID: PMC9042589
- DOI: 10.1039/d1ra06567j
Chemosensors based on N-heterocyclic dyes: advances in sensing highly toxic ions such as CN- and Hg2
Abstract
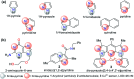
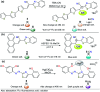
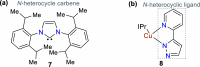
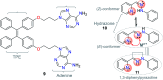
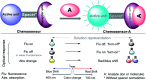
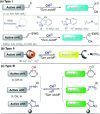
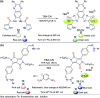
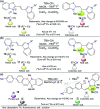
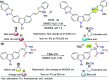
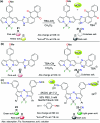
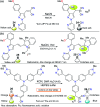
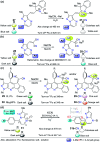
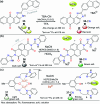
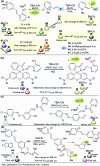
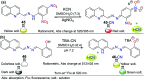
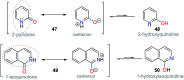
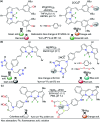
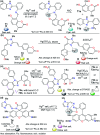
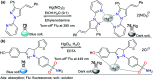
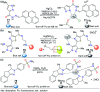
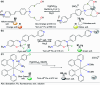
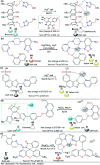
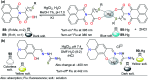
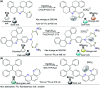
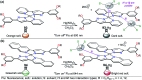
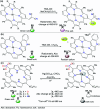
CN- and Hg2+ ions are harmful to both the environment and human health, even at trace levels. Thus, alternative methods for their detection and quantification are highly desirable given that the traditional monitoring systems are expensive and require qualified personnel. Optical chemosensors (probes) have revolutionized the sensing of different species due to their high specificity and sensitivity, corresponding with their modular design. They have also been used in aqueous media and different pH ranges, facilitating their applications in various samples. The design of molecular probes is based on organic dyes, where the key species are N-heterocyclic compounds (NHCs) due to their proven photophysical properties, biocompatibility, and synthetic versatility, which favor diverse applications. Accordingly, this review aims to provide an overview of the reports from 2016 to 2021, in which fluorescent probes based on five- and six-membered N-heterocycles are used for the detection of CN- and Hg2+ ions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



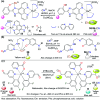
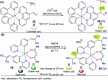
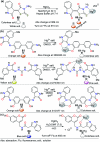
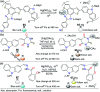




References
-
- Alvarez-Builla Julio J., Vaquero J. J. and Barluenga J., Modern Heterocyclic Chemistry, Wiley-VCH Verlag & Co. KGaA, Weinheim, Germany, 2011, vol. 4
-
- Castillo J.-C. Portilla J. Targets Heterocycl. Syst. 2019;22:194–223.
Publication types
LinkOut - more resources
Full Text Sources

