Faster Growth Enhances Low Carbon Fuel and Chemical Production Through Gas Fermentation
- PMID: 35497340
- PMCID: PMC9039284
- DOI: 10.3389/fbioe.2022.879578
Faster Growth Enhances Low Carbon Fuel and Chemical Production Through Gas Fermentation
Abstract
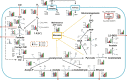
Gas fermentation offers both fossil carbon-free sustainable production of fuels and chemicals and recycling of gaseous and solid waste using gas-fermenting microbes. Bioprocess development, systems-level analysis of biocatalyst metabolism, and engineering of cell factories are advancing the widespread deployment of the commercialised technology. Acetogens are particularly attractive biocatalysts but effects of the key physiological parameter-specific growth rate (μ)-on acetogen metabolism and the gas fermentation bioprocess have not been established yet. Here, we investigate the μ-dependent bioprocess performance of the model-acetogen Clostridium autoethanogenum in CO and syngas (CO + CO2+H2) grown chemostat cultures and assess systems-level metabolic responses using gas analysis, metabolomics, transcriptomics, and metabolic modelling. We were able to obtain steady-states up to μ ∼2.8 day-1 (∼0.12 h-1) and show that faster growth supports both higher yields and productivities for reduced by-products ethanol and 2,3-butanediol. Transcriptomics data revealed differential expression of 1,337 genes with increasing μ and suggest that C. autoethanogenum uses transcriptional regulation to a large extent for facilitating faster growth. Metabolic modelling showed significantly increased fluxes for faster growing cells that were, however, not accompanied by gene expression changes in key catabolic pathways for CO and H2 metabolism. Cells thus seem to maintain sufficient "baseline" gene expression to rapidly respond to CO and H2 availability without delays to kick-start metabolism. Our work advances understanding of transcriptional regulation in acetogens and shows that faster growth of the biocatalyst improves the gas fermentation bioprocess.
Keywords: Clostridium autoethanogenum; acetogen; carbon recycling; chemostat; gas fermentation; genome-scale metabolic modelling; metabolomics; transcriptomics.
Copyright © 2022 de Lima, Ingelman, Brahmbhatt, Reinmets, Barry, Harris, Marcellin, Köpke and Valgepea.
Conflict of interest statement
LanzaTech has interest in commercial gas fermentation with C. autoethanogenum. AH and MK are employees of LanzaTech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

