Cu/TEMPO catalyzed dehydrogenative 1,3-dipolar cycloaddition in the synthesis of spirooxindoles as potential antidiabetic agents
- PMID: 35497611
- PMCID: PMC9050786
- DOI: 10.1039/d0ra01553a
Cu/TEMPO catalyzed dehydrogenative 1,3-dipolar cycloaddition in the synthesis of spirooxindoles as potential antidiabetic agents
Abstract
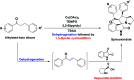
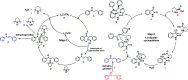
A series of spiro-[indoline-3,3'-pyrrolizin/pyrrolidin]-2-ones, 4, 5 and 6 were synthesized in a sequential manner from Cu-TEMPO catalyzed dehydrogenation of alkylated ketones, 1 followed by 1,3-dipolar cycloaddition of azomethine ylides via decarboxylative condensation of isatin, 2 and l-proline/sarcosine, 3 in high regioselectivities and yields. The detailed mechanistic studies were performed to identify the reaction intermediates, which revealed that the reaction proceeds via dehydrogenative cycloaddition. Additionally, the regio and stereochemistry of the synthesized derivatives were affirmed by 2D NMR spectroscopic studies. The synthesized derivatives were explored further with molecular docking, in vitro antioxidant, and anti-diabetic activities.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
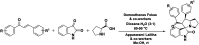
Similar articles
-
A Diastereoselective Synthesis of Dispiro[oxindole-cyclohexanone]pyrrolidines by 1,3-Dipolar Cycloaddition.Molecules. 2017 Dec 4;22(12):2134. doi: 10.3390/molecules22122134. Molecules. 2017. PMID: 29207519 Free PMC article.
-
An efficient synthesis of novel dispirooxindole derivatives via one-pot three-component 1,3-dipolar cycloaddition reactions.Molecules. 2012 Oct 26;17(11):12704-17. doi: 10.3390/molecules171112704. Molecules. 2012. PMID: 23103534 Free PMC article.
-
Catalytic enantioselective 1,3-dipolar cycloadditions of azomethine ylides for biology-oriented synthesis.Acc Chem Res. 2014 Apr 15;47(4):1296-310. doi: 10.1021/ar400286b. Epub 2014 Mar 22. Acc Chem Res. 2014. PMID: 24730692 Free PMC article.
-
1,3-Dipolar cycloaddition reactions of isatin-derived azomethine ylides for the synthesis of spirooxindole and indole-derived scaffolds: recent developments.Mol Divers. 2023 Oct;27(5):2365-2397. doi: 10.1007/s11030-022-10510-9. Epub 2022 Aug 4. Mol Divers. 2023. PMID: 35925529 Review.
-
1,3-Dipolar Cycloaddition Reactions of Azomethine Ylides with Carbonyl Dipolarophiles Yielding Oxazolidine Derivatives.Molecules. 2016 Jul 23;21(8):935. doi: 10.3390/molecules21080935. Molecules. 2016. PMID: 27455230 Free PMC article. Review.
Cited by
-
Transition metal-catalyzed synthesis of spirooxindoles.RSC Adv. 2021 Feb 10;11(13):7146-7179. doi: 10.1039/d1ra00139f. eCollection 2021 Feb 10. RSC Adv. 2021. PMID: 35423236 Free PMC article. Review.
-
Profiling of Potential Anti-Diabetic Active Compounds in White Tea: An Integrated Study of Polyphenol-Targeted Metabolomics, Network Pharmacology, and Computer Simulation.Foods. 2024 Oct 22;13(21):3354. doi: 10.3390/foods13213354. Foods. 2024. PMID: 39517138 Free PMC article.
-
Benzodioxole grafted spirooxindole pyrrolidinyl derivatives: synthesis, characterization, molecular docking and anti-diabetic activity.RSC Adv. 2022 Aug 25;12(37):24192-24207. doi: 10.1039/d2ra04452h. eCollection 2022 Aug 22. RSC Adv. 2022. PMID: 36128541 Free PMC article.
-
Selective and Reversible 1,3-Dipolar Cycloaddition of 2-(2-Oxoindoline-3-ylidene)acetates with Nitrones in the Synthesis of Functionalized Spiroisoxazolidines.Int J Mol Sci. 2022 Oct 20;23(20):12639. doi: 10.3390/ijms232012639. Int J Mol Sci. 2022. PMID: 36293495 Free PMC article.
-
Synthesis of novel rapanone derivatives via organocatalytic reductive C-alkylation: biological evaluation of antioxidant properties, in vivo zebrafish embryo toxicity, and docking studies.RSC Med Chem. 2023 Dec 4;15(2):623-635. doi: 10.1039/d3md00564j. eCollection 2024 Feb 21. RSC Med Chem. 2023. PMID: 38389875 Free PMC article.
References
-
- Gülçin I. Elmastaşan M. Dandia A. Jain A. K. Laxkar A. K. RSC Adv. 2013;3:8422–8430. doi: 10.1039/C3RA00170A. - DOI
LinkOut - more resources
Full Text Sources

