A study on the mechanism of oxidized quinoline removal from acid solutions based on persulfate-iron systems
- PMID: 35497624
- PMCID: PMC9051261
- DOI: 10.1039/c9ra10556e
A study on the mechanism of oxidized quinoline removal from acid solutions based on persulfate-iron systems
Abstract
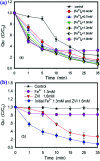
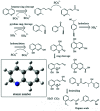
Quinoline (Qu) and its derivatives have been widely regarded as hazardous pollutants in the world because of their acute toxicity to humans and animals, and potential carcinogenic risks. In this study, a novel sulfate radical system co-activated by ferrous and ZVI was developed to remove Qu from acidic solutions. The optimal ratio of ferrous and ZVI in the system and the mechanism of Qu removal from acidic solutions are also explored. The ZVI can initiate activation using hydrogen ions, which are released from the reaction of Fe2+, organics and PS in acidic solutions. This may dramatically improve the overall removal efficiency of Qu. The results indicated that the initial removal rate of Qu increases from 85.8% to 92.9%. The cleavage pathway of Qu is speculated by Frontier molecular orbital (FMO) theory and verified by GC/MS analysis.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Chen J. Wang X. Huang Y. Lv S. Cao X. Yun J. Cao D. Engineered Science. 2019;5:30–38.
-
- Luo X. Pan Z. Pei F. Jin Z. Miao K. Yang P. Feng G. J. Ind. Eng. Chem. 2018;59:410–415. doi: 10.1016/j.jiec.2017.10.052. - DOI
-
- Shi X. Wang C. Ma Y. Liu H. Wu S. Shao Q. Guo Z. Powder Technol. 2019;356:726–734. doi: 10.1016/j.powtec.2019.09.002. - DOI
-
- Rameshraja D. Srivastava V. C. Kushwaha J. P. Mall I. D. Chem. Pap. 2018;72:617–628. doi: 10.1007/s11696-017-0321-6. - DOI
-
- Guerra-Rodríguez S. Rodríguez E. Singh D. N. Rodríguez-Chueca J. Water. 2018;10:1828. doi: 10.3390/w10121828. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

