The Prevalence of Pharmacogenomics Variants and Their Clinical Relevance Among the Pakistani Population
- PMID: 35497687
- PMCID: PMC9047794
- DOI: 10.1177/11769343221095834
The Prevalence of Pharmacogenomics Variants and Their Clinical Relevance Among the Pakistani Population
Abstract
Background: Pharmacogenomics (PGx), forming the basis of precision medicine, has revolutionized traditional medical practice. Currently, drug responses such as drug efficacy, drug dosage, and drug adverse reactions can be anticipated based on the genetic makeup of the patients. The pharmacogenomic data of Pakistani populations are limited. This study investigates the frequencies of pharmacogenetic variants and their clinical relevance among ethnic groups in Pakistan.
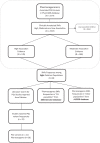
Methods: The Pharmacogenomics Knowledge Base (PharmGKB) database was used to extract pharmacogenetic variants that are involved in medical conditions with high (1A + 1B) to moderate (2A + 2B) clinical evidence. Subsequently, the allele frequencies of these variants were searched among multiethnic groups of Pakistan (Balochi, Brahui, Burusho, Hazara, Kalash, Pashtun, Punjabi, and Sindhi) using the 1000 Genomes Project (1KGP) and ALlele FREquency Database (ALFRED). Furthermore, the published Pharmacogenomics literature on the Pakistani population was reviewed in PubMed and Google Scholar.
Results: Our search retrieved (n = 29) pharmacogenetic genes and their (n = 44) variants with high to moderate evidence of clinical association. These pharmacogenetic variants correspond to drug-metabolizing enzymes (n = 22), drug-metabolizing transporters (n = 8), and PGx gene regulators, etc. (n = 14). We found 5 pharmacogenetic variants present at >50% among 8 ethnic groups of Pakistan. These pharmacogenetic variants include CYP2B6 (rs2279345, C; 70%-86%), CYP3A5 (rs776746, C; 64%-88%), FLT3 (rs1933437, T; 54%-74%), CETP (rs1532624, A; 50%-70%), and DPP6 (rs6977820, C; 61%-86%) genes that are involved in drug response for acquired immune deficiency syndrome, transplantation, cancer, heart disease, and mental health therapy, respectively.
Conclusions: This study highlights the frequency of important clinical pharmacogenetic variants (1A, 1B, 2A, and 2B) among multi-ethnic Pakistani populations. The high prevalence (>50%) of single nucleotide pharmacogenetic variants may contribute to the drug response/diseases outcome. These PGx data could be used as pharmacogenetic markers in the selection of appropriate therapeutic regimens for specific ethnic groups of Pakistan.
Keywords: Pakistan; Pharmacogenomics; allele frequency; drug-metabolizing enzymes; drug-transporters; pharmacogenetic variants.
© The Author(s) 2022.
Conflict of interest statement
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
The prevalence of pharmacogenetic variants of vitamin K epoxide reductase complex subunit 1 gene (rs9923231), cytochrome P450 family 2 subfamily C member 9 gene (rs1799853) and cytochrome P450 family 3 subfamily-A member-5 gene (rs776746) among 13 ethnic groups of Pakistan.Mol Biol Rep. 2023 May;50(5):4017-4027. doi: 10.1007/s11033-023-08304-9. Epub 2023 Feb 27. Mol Biol Rep. 2023. PMID: 36849858
-
Prevalence of exposure to pharmacogenetic drugs by the Saudis treated at the health care centers of the Ministry of National Guard.Saudi Pharm J. 2022 Aug;30(8):1181-1192. doi: 10.1016/j.jsps.2022.06.013. Epub 2022 Jun 22. Saudi Pharm J. 2022. PMID: 36164570 Free PMC article.
-
Genetic analysis of pharmacogenomic VIP variants in the Wa population from Yunnan Province of China.BMC Genom Data. 2021 Nov 19;22(1):51. doi: 10.1186/s12863-021-00999-8. BMC Genom Data. 2021. PMID: 34798807 Free PMC article.
-
Pharmacogenomic Profiling of ADME Gene Variants: Current Challenges and Validation Perspectives.High Throughput. 2018 Dec 18;7(4):40. doi: 10.3390/ht7040040. High Throughput. 2018. PMID: 30567415 Free PMC article. Review.
-
Evidence and resources to implement pharmacogenetic knowledge for precision medicine.Am J Health Syst Pharm. 2016 Dec 1;73(23):1977-1985. doi: 10.2146/ajhp150977. Am J Health Syst Pharm. 2016. PMID: 27864205 Free PMC article. Review.
References
-
- The Clinical Pharmacogenetics Implementation Consortium (CPIC®). 2019. Accessed July 20, 2019. https://cpicpgx.org
-
- The Canadian Pharmacogenomics Network for Drug Safety. 2019. Accessed July 20, 2019. http://cpnds.ubc.ca/
LinkOut - more resources
Full Text Sources
Miscellaneous