The C-H activated controlled mono- and di-olefination of arenes in ionic liquids at room temperature
- PMID: 35497718
- PMCID: PMC9048982
- DOI: 10.1039/c9ra09736h
The C-H activated controlled mono- and di-olefination of arenes in ionic liquids at room temperature
Abstract
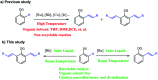
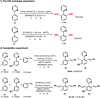
In this study, controlled mono and di-olefination of arenes was first realized at room temperature via the C-H bond activation in ionic liquids, probably due to the positive effects of ionic liquids. It is an energy-saving routes in industrial production without the need for heating equipment. Different catalysts were screened, and it was found that [Ru(p-cymene)Cl2]2 generated mono-olefinated products predominantly while [Cp*RhCl2]2 selectively gave di-olefinated products. These catalysts ([BMIM]NTf2 and [BMIM]PF6) as green and recyclable reaction media are highly efficient under mild conditions. This reaction process can avoid any volatile and environmentally toxic organic solvents, and is much safer without the need for pressure-tight equipment. A wide substrate scope with good yields and satisfactory selectivity was achieved. The reactions can be scaled up to gram-scale. Furthermore, an expensive rhodium/ruthenium catalytic system was recycled for at least 6 times with consistently high catalytic activity, which was economical and environmental friendly from an industrial point of view. According to the mechanistic study, the C-H bond cleavage was probably achieved via the concerted metalation-deprotonation. This technique can be applied in the synthesis of various valuable unsaturated aromatic compounds and shows a great potential for industrial production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Li B. Dixneuf P. H. Chem. Soc. Rev. 2013;42:5744. doi: 10.1039/C3CS60020C. - DOI - PubMed
- Wencel-Delord J. Droege T. Liu F. Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/C1CS15083A. - DOI - PubMed
- Yeung C. S. Dong V. M. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. - DOI - PubMed
- Zhao B. Shi Z. Yuan Y. Chem. Rec. 2016;16:886. doi: 10.1002/tcr.201500241. - DOI - PubMed
-
- Chen G. Gong W. Zhuang Z. Science. 2016;353:1023. doi: 10.1126/science.aaf4434. - DOI - PMC - PubMed
- Sang R. Zheng Y. Zhang H. et al. . Org. Chem. Front. 2018;5:648. doi: 10.1039/C7QO00945C. - DOI
- Zhang C. Pu Y. Yu Z. et al. . Org. Chem. Front. 2018;5:1288. doi: 10.1039/C8QO00009C. - DOI
- Zhang H. Jing L. Zheng Y. et al. . Eur. J. Org. Chem. 2018;2018:723. doi: 10.1002/ejoc.201701476. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

