Past, Present, and Future of Phosphate Management
- PMID: 35497793
- PMCID: PMC9039476
- DOI: 10.1016/j.ekir.2022.01.1055
Past, Present, and Future of Phosphate Management
Abstract
Cardiovascular (CV) disease (CVD) accounts for >50% of deaths with known causes in patients on dialysis. Elevated serum phosphorus levels are an important nontraditional risk factor for bone mineral disease and CVD in patients with chronic kidney disease (CKD). Given that phosphorus concentrations drive other disorders associated with increased CV risk (e.g., endothelial dysfunction, vascular calcification, fibroblast growth factor-23, parathyroid hormone), phosphate is a logical target to improve CV health. Phosphate binders are the only pharmacologic treatment approved for hyperphosphatemia. Although their safety has improved since inception, the mechanism of action leads to characteristics that make ingestion difficult and unpleasant; large pill size, objectionable taste, and multiple pills required for each meal and snack make phosphate binders a burden. Side effects, especially those affecting the gastrointestinal (GI) system, are common with binders, often leading to treatment discontinuation. The presence of "hidden" phosphates in processed foods and certain medications makes phosphate management even more challenging. Owing to these significant issues, most patients on dialysis are not consistently achieving and maintaining target phosphorus concentrations of <5.5 mg/dl, let alone more normal levels of <4.5 mg/dl, indicating novel approaches to improve phosphate management and CV health are needed. Several new nonbinder therapies that target intestinal phosphate absorption pathways have been developed. These include EOS789, which acts on the transcellular pathway, and tenapanor, which targets the dominant paracellular pathway. As observational evidence has established a strong association between phosphorus concentration and clinical outcomes, such as mortality, phosphate is an important target for improving the health of patients with CKD and end-stage kidney disease (ESKD).
Keywords: chronic kidney disease; end stage renal disease; hyperphosphatemia; phosphate binder; phosphate management; serum phosphorus.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures
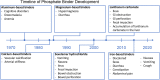
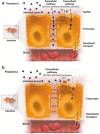
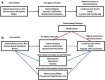
References
-
- United States Renal Data System 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://adr.usrds.org/2020/ Published 2020.
-
- United States Renal Data System 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. https://www.usrds.org/annual-data-report/ Published 2016.
-
- Number of MBD markers out of range. vol 2021. DOPPS; 2021. https://www.dopps.org/DPM-HD/DPMSlideBrowser.aspx?type=Topic&id=11
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

