Thymoglobulin Versus Alemtuzumab Versus Basiliximab Kidney Transplantation From Donors After Circulatory Death
- PMID: 35497810
- PMCID: PMC9039467
- DOI: 10.1016/j.ekir.2022.01.1042
Thymoglobulin Versus Alemtuzumab Versus Basiliximab Kidney Transplantation From Donors After Circulatory Death
Abstract
Introduction: The Campath, Calcineurin inhibitor (CNI) reduction, and Chronic allograft nephropathy (3C), a study comparing alemtuzumab versus basiliximab induction immunosuppression in kidney transplants, has found lower acute rejection rate with alemtuzumab but same graft survival. The aim of the current study is to evaluate the effect of induction immunosuppression (thymoglobulin, alemtuzumab, basiliximab) on the outcome of kidneys of donors after circulatory death (DCD).
Methods: Data of the 274 DCD patients of the 3C obtained from the sponsor were compounded with the 140 DCD patients who received thymoglobulin in a single center with the same entry criteria as the 3C, giving 414 patients on 3 induction regimes.
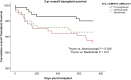
Results: There were more male donors (P < 0.05) and human leukocyte antigen and DR mismatched patients in the thymoglobulin group (P < 0.001). Death-censored graft survival at 6 months was 98.6% in the thymoglobulin, 95.5% in the alemtuzumab (P = 0.08), and 95.7% in the basiliximab group (P = 0.09) and at 2 years 97.9% versus 94.8% (P = 0.13, hazard ratio [HR] 2.8, 95% CI 0.7-10.9) versus 94.3% (P = 0.06, HR 3.5, 95% CI 0.9-13.6), respectively.The 2-year overall graft survival was 95% in the thymoglobulin versus 88% in the alemtuzumab (unadjusted P = 0.038, adjusted HR 2.4, 95% CI 0.99-5.9) and 91.4% in the basiliximab group (P = 0.21). The 2-year patient survival was numerically less in the alemtuzumab compared with the thymoglobulin group (91.8% vs. 97.1%, P = 0.052, HR 2.90, 95% CI 0.93-9.2). Acute rejection was 17% in the basiliximab, 4.3% in the thymoglobulin, and 6% in the alemtuzumab group (P < 0.001).
Conclusion: In DCD transplants, thymoglobulin induction may provide advantage over alemtuzumab in patient survival and the same advantage as alemtuzumab over basiliximab in terms of acute rejection. Differing maintenance immunosuppression may contribute to the difference found.
Keywords: alemtuzumab; basiliximab; donation after circulatory death; immunosuppression; kidney transplantation; thymoglobulin.
© 2022 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
-
- NHSBT Transplant activity in the UK—activity report 2018–19. NHSBT. 2019. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/16537/organ-d... Accessed January 3, 2022. Available at:
-
- NHSBT Organ Donation and Transplantation Activity Report 2019–21. https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/19220/activit... Accessed January 3, 2022. Available at:
LinkOut - more resources
Full Text Sources

