Disruption of Pituitary Gonadotrope Activity in Male Rats After Short- or Long-Term High-Fat Diets Is Not Associated With Pituitary Inflammation
- PMID: 35498414
- PMCID: PMC9043610
- DOI: 10.3389/fendo.2022.877999
Disruption of Pituitary Gonadotrope Activity in Male Rats After Short- or Long-Term High-Fat Diets Is Not Associated With Pituitary Inflammation
Abstract
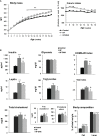
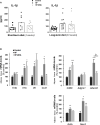
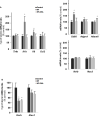
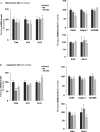
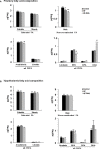
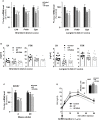
Overnutrition is associated with the activation of inflammatory pathways in metabolically linked organs and an early hypothalamic inflammation is now known to disrupt the central control of metabolic function. Because we demonstrated that fatty acids (FA) target the pituitary and affect gonadotropin synthesis, we asked whether overnutrition induces pituitary inflammation that may contribute to obesity-associated disorders in the control of reproduction. We analyzed pituitary inflammation and hypothalamic-pituitary-testicular axis in male rats fed a short- (4 weeks) or long-term (20 weeks) high-fat diet. The effect of diet enrichment with the ω3 polyunsaturated FA, DHA, was also analyzed. After only 4 weeks and before weight gain of rats, high-fat diet caused a significant decrease in pituitary gonadotropin and hypothalamic GnRH transcript levels despite unchanged testosterone and inhibin B levels. Contrasting with the hypothalamus, there was no concomitant increases in gene expression of pituitary inflammatory mediators and even a reduction of prototypical cytokines such as interleukin-1β and TNF-α. No inflammation was still detected in the pituitary after 20 weeks although gonadotropin transcripts and circulating levels were still altered. Gonadotropins were the only pituitary hormones remaining affected at this stage of the regimen, underlying a differential susceptibility of pituitary lineages to metabolic disorders. DHA enrichment of the diet did not prevent alterations of gonadotrope activity due to either a long- or a short-term high-fat diet although it blocked early hypothalamic inflammation and attenuated several metabolic effects. Taken together, our findings suggest that high-fat diet-induced defects in gonadotrope activity in male rats occurred despite a lack of pituitary inflammation.
Keywords: fatty acids; gonadotropin; high-fat diet; inflammation; omega 3; pituitary.
Copyright © 2022 Garrel, Rouch, L’Hôte, Tazi, Kassis, Giton, Dairou, Dournaud, Gressens, Magnan, Cruciani-Guglielmacci and Cohen-Tannoudji.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Fernandez-Fernandez R, Tena-Sempere M, Navarro VM, Barreiro ML, Castellano JM, Aguilar E, et al. . Effects of Ghrelin Upon Gonadotropin-Releasing Hormone and Gonadotropin Secretion in Adult Female Rats: In Vivo and In Vitro Studies. Neuroendocrinology (2005) 82(5-6):245–55. doi: 10.1159/000092753 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

