Colistin Loading Dose in Septic Patients with Gram Negative Infections
- PMID: 35498632
- PMCID: PMC9048962
- DOI: 10.2147/IDR.S361244
Colistin Loading Dose in Septic Patients with Gram Negative Infections
Abstract
Purpose: Intravenous (IV) colistin is commonly used to treat multidrug-resistant gram-negative infections. It is primarily eliminated renally and may induce acute kidney injury (AKI) at a rate of up to 53%. Consequently, septic patients who require colistin administration have an additional risk of developing AKI. The aim of this study is to investigate clinical failure and AKI predictors for septic patients treated with IV colistin.
Methods: This retrospective cohort study was conducted at a tertiary teaching hospital in Saudi Arabia. Adult septic patients with suspected or confirmed gram-negative infections who received colistin admitted to the hospital between May 2016 and December 2020 were screened after obtaining IRB approval. AKI was defined based on the AKI Network criteria. We investigated the incidence of clinical failure based on colistin dosing and AKI risk factors, such as the development of septic shock, severity of illness, and medication co-administration using a multiple logistic regression model.
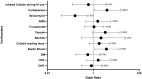
Results: After screening 163 patients, 103 patients were included in the analysis. No difference was observed between the colistin dosing strategies for clinical failure. Of the included predictors, development of septic shock (OR: 3.75; 95% CI 1.18-13.15), carbapenem co-administration (OR, 3.96; 95% CI, 1.134-15.57) were associated with an increased risk of AKI. The other factors were not significant predictors.
Conclusion: Clinical failure was not affected by colistin dosing strategies in our cohort of patients with sepsis. Moreover, the co-administration of carbapenems and the development of septic shock may increase the risk of inducing AKI in adult septic patients treated with IV colistin. Further studies are required to confirm these findings.
Keywords: colistin; gram-negative; infection; loading dose; renal failure; sepsis.
© 2022 Alshaya et al.
Conflict of interest statement
This original research was accepted to be presented at the Society of Critical Care Medicine’s (SCCM) 2022 Critical Care Congress and the abstract is currently published in Critical Care Medicine (https://journals.lww.com/ccmjournal/fulltext/2022/01001/1330__predictors_for_acute_kidney_injury_for.1296.aspx). The authors report no conflicts of interest for this work.
Figures
References
-
- Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical