Osmotic Adjustment and Antioxidant System Regulated by Nitrogen Deposition Improve Photosynthetic and Growth Performance and Alleviate Oxidative Damage in Dwarf Bamboo Under Drought Stress
- PMID: 35498701
- PMCID: PMC9047053
- DOI: 10.3389/fpls.2022.819071
Osmotic Adjustment and Antioxidant System Regulated by Nitrogen Deposition Improve Photosynthetic and Growth Performance and Alleviate Oxidative Damage in Dwarf Bamboo Under Drought Stress
Abstract
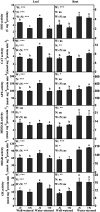
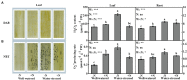
Dwarf bamboo (Fargesia denudata) is a staple food for the endangered giant pandas and plays a critical role in the sub-alpine ecosystem. Characterized by shallow roots and expeditious growth, it is exceedingly susceptible to drought stress and nitrogen (N) deposition in the context of a changing global environment. However, a comprehensive picture about the interactive response mechanism of dwarf bamboo to the two factors, water regime and N deposition, is far from being given. Therefore, a completely randomized design with two factors of water regimes (well-watered and water-stressed) and N deposition levels (with and without N addition) of F. denudata was conducted. In view of the obtained results, drought stress had an adverse impact on F. denudata, showing that it destroyed ultrastructure integrity and induced oxidative damage and restricted water status in leaves and roots, as well as declined photosynthetic efficiency in leaves, especially in N non-deposition plants. Nevertheless, F. denudata significantly increased heat dissipation in leaves, regulated antioxidant enzymes activities, antioxidants contents, and osmoregulation substances concentrations in leaves and roots, as well as shifted biomass partitioning in response to drought stress. However, regardless of water availability, N deposition maintained better ultrastructure in leaves and roots, resulting in superior photosynthesis and growth of F. denudata. Additionally, although N deposition did not cause oxidative damage in well-watered plants, ameliorated the effects of drought stress on F. denudata through co-deploying heat dissipation in leaves, the antioxidant system in roots as well as osmotic adjustment in leaves and roots. Noticeably, the leaves and roots of F. denudata expressed quite distinct acclimation responses to drought resistance under N deposition.
Keywords: Fargesia denudata; antioxidative defense system; drought stress; nitrogen deposition; osmotic adjustment.
Copyright © 2022 Wu, Tian, Ren and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Agami R. A., Alamri S. A., Abd El-Mageed T., Abousekken M., Hashem M. (2018). Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 210, 261–270. doi: 10.1016/j.agwat.2018.08.034 - DOI
-
- Ali Z., Golombek S. (2016). Effect of drought and nitrogen availability on osmotic adjustment of five pearl millet cultivars in the vegetative growth stage. J. Agron. Crop Sci. 202, 433–444. doi: 10.1111/jac.12163 - DOI
-
- Arrigoni O., Dipierro S., Borraccino G. (1981). Ascorbate free radical reductase, a key enzyme of the ascorbic acid system. FEBS Lett. 125, 242–244. doi: 10.1016/0014-5793(81)80729-6 - DOI
-
- Azzeme A. M., Abdullah S. N. A., Aziz M. A., Wahab P. E. M. (2016). Oil palm leaves and roots differ in physiological response, antioxidant enzyme activities and expression of stress-responsive genes upon exposure to drought stress. Acta Physiol. Plant. 38, 52. doi: 10.1007/s11738-016-2073-2 - DOI
LinkOut - more resources
Full Text Sources

