Defined Antigen Skin Test for Bovine Tuberculosis Retains Specificity on Revaccination With Bacillus Calmette-Guérin
- PMID: 35498753
- PMCID: PMC9043861
- DOI: 10.3389/fvets.2022.814227
Defined Antigen Skin Test for Bovine Tuberculosis Retains Specificity on Revaccination With Bacillus Calmette-Guérin
Abstract
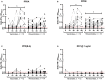
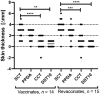
The Bacillus Calmette-Guérin (BCG) vaccination provides partial protection against, and reduces severity of pathological lesions associated with bovine tuberculosis (bTB) in cattle. Accumulating evidence also suggests that revaccination with BCG may be needed to enhance the duration of immune protection. Since BCG vaccine cross-reacts with traditional tuberculin-based diagnostic tests, a peptide-based defined antigen skin test (DST) comprising of ESAT-6, CFP-10, and Rv3615c to detect the infected among the BCG-vaccinated animals (DIVA) was recently described. The DST reliably identifies bTB-infected animals in experimental challenge models and in natural infection settings, and differentiated these from animals immunized with a single dose of BCG in both skin tests and interferon-gamma release assay (IGRA). The current investigation sought to assess the diagnostic specificity of DST in calves (Bos taurus ssp. taurus × B. t. ssp. indicus; n = 15) revaccinated with BCG 6 months after primary immunization. The results show that none of the 15 BCG-revaccinated calves exhibited a delayed hypersensitivity response when skin tested with DST 61 days post-revaccination, suggesting 100% diagnostic specificity (one-tailed lower 95% CI: 82). In contrast, 8 of 15 (diagnostic specificity = 47%; 95% CI: 21, 73) BCG-revaccinated calves were positive per the single cervical tuberculin (SCT) test using bovine tuberculin. Together, these results show that the DST retains its specificity even after revaccination with BCG and confirms the potential for implementation of BCG-based interventions in settings where test-and-slaughter are not economically or culturally feasible.
Keywords: Bacillus Calmette-Guérin (BCG) vaccine; DIVA; DST; bovine tuberculosis (bTB); specificity.
Copyright © 2022 Subramanian, Srinivasan, Ramaiyan Selvaraju, Vinoli, Selladurai, Ramasamy, Kumaragurubaran, Bakker, Vordermeier, Kapur and Gopal.
Conflict of interest statement
APHA (MV) and Penn State (SSr and VK) have filed a patent application for DST (U.S. Patent Application 17/602,628). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Naugle AL, Schoenbaum MA, Hench CW, Henderson OL, Shere J. Bovine Tuberculosis Eradication in the United States. Hoboken: Wiley; (2014).
-
- Olmstead AL, Rhode PW. An impossible undertaking: the eradication of bovine tuberculosis in the United States. J Econ Hist. (2004) 64:734–72. 10.1017/S0022050704002955 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

