HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy
- PMID: 35499076
- PMCID: PMC9057582
- DOI: 10.1172/JCI156774
HIF inhibitor 32-134D eradicates murine hepatocellular carcinoma in combination with anti-PD1 therapy
Abstract
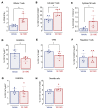
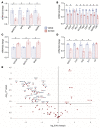
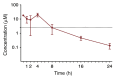
Hepatocellular carcinoma (HCC) is a major cause of cancer mortality worldwide and available therapies, including immunotherapies, are ineffective for many patients. HCC is characterized by intratumoral hypoxia, and increased expression of hypoxia-inducible factor 1α (HIF-1α) in diagnostic biopsies is associated with patient mortality. Here we report the development of 32-134D, a low-molecular-weight compound that effectively inhibits gene expression mediated by HIF-1 and HIF-2 in HCC cells, and blocks human and mouse HCC tumor growth. In immunocompetent mice bearing Hepa1-6 HCC tumors, addition of 32-134D to anti-PD1 therapy increased the rate of tumor eradication from 25% to 67%. Treated mice showed no changes in appearance, behavior, body weight, hemoglobin, or hematocrit. Compound 32-134D altered the expression of a large battery of genes encoding proteins that mediate angiogenesis, glycolytic metabolism, and responses to innate and adaptive immunity. This altered gene expression led to significant changes in the tumor immune microenvironment, including a decreased percentage of tumor-associated macrophages and myeloid-derived suppressor cells, which mediate immune evasion, and an increased percentage of CD8+ T cells and natural killer cells, which mediate antitumor immunity. Taken together, these preclinical findings suggest that combining 32-134D with immune checkpoint blockade may represent a breakthrough therapy for HCC.
Keywords: Cancer immunotherapy; Liver cancer; Oncology; Therapeutics; Transcription.
Conflict of interest statement
Figures


Comment in
-
Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy.J Clin Invest. 2022 May 2;132(9):e159473. doi: 10.1172/JCI159473. J Clin Invest. 2022. PMID: 35499071 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

