Labile iron accumulation augments T follicular helper cell differentiation
- PMID: 35499081
- PMCID: PMC9057622
- DOI: 10.1172/JCI159472
Labile iron accumulation augments T follicular helper cell differentiation
Abstract
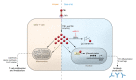
T follicular helper (Tfh) cells are a subset of CD4+ T cells that are essential in the pathogenesis of systemic lupus erythematosus (SLE). Notably, iron is required for activated CD4+ T lymphocytes to sustain high proliferation and metabolism. In this issue of the JCI, Gao et al. showed that CD4+ T cells from patients with SLE accumulated iron, augmenting their differentiation into Tfh cells and correlating with disease activity. Using human cells and murine models, the authors demonstrated that miR-21 was overexpressed in lupus T cells and inhibited 3-hydroxybutyrate dehydrogenase-2 (BDH2). The subsequent loss of BDH2 drove labile iron to accumulate in the cytoplasm and promoted TET enzyme activity, BCL6 gene demethylation, and Tfh cell differentiation. This work identifies a role for iron in CD4+ T cell biology and the development of pathogenic effectors in SLE. We await future investigations that could determine whether modulating iron levels could regulate Tfh cells in human health and disease.
Conflict of interest statement
Figures
Comment on
-
Iron-dependent epigenetic modulation promotes pathogenic T cell differentiation in lupus.J Clin Invest. 2022 May 2;132(9):e152345. doi: 10.1172/JCI152345. J Clin Invest. 2022. PMID: 35499082 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

