Loss of the collagen IV modifier prolyl 3-hydroxylase 2 causes thin basement membrane nephropathy
- PMID: 35499085
- PMCID: PMC9057608
- DOI: 10.1172/JCI147253
Loss of the collagen IV modifier prolyl 3-hydroxylase 2 causes thin basement membrane nephropathy
Abstract
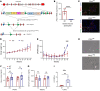
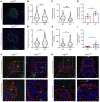
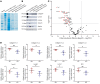
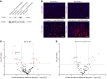
The glomerular filtration barrier (GFB) produces primary urine and is composed of a fenestrated endothelium, a glomerular basement membrane (GBM), podocytes, and a slit diaphragm. Impairment of the GFB leads to albuminuria and microhematuria. The GBM is generated via secreted proteins from both endothelial cells and podocytes and is supposed to majorly contribute to filtration selectivity. While genetic mutations or variations of GBM components have been recently proposed to be a common cause of glomerular diseases, pathways modifying and stabilizing the GBM remain incompletely understood. Here, we identified prolyl 3-hydroxylase 2 (P3H2) as a regulator of the GBM in an a cohort of patients with albuminuria. P3H2 hydroxylates the 3' of prolines in collagen IV subchains in the endoplasmic reticulum. Characterization of a P3h2ΔPod mouse line revealed that the absence of P3H2 protein in podocytes induced a thin basement membrane nephropathy (TBMN) phenotype with a thinner GBM than that in WT mice and the development of microhematuria and microalbuminuria over time. Mechanistically, differential quantitative proteomics of the GBM identified a significant decrease in the abundance of collagen IV subchains and their interaction partners in P3h2ΔPod mice. To our knowledge, P3H2 protein is the first identified GBM modifier, and loss or mutation of P3H2 causes TBMN and focal segmental glomerulosclerosis in mice and humans.
Keywords: Chronic kidney disease; Collagens; Monogenic diseases; Nephrology.
Conflict of interest statement
Figures




Comment in
-
Thin basement membrane lesion is not only a collagen IV nephropathy: do not underestimate "decorative" additions to collagens.Kidney Int. 2022 Dec;102(6):1203-1205. doi: 10.1016/j.kint.2022.08.014. Epub 2022 Aug 27. Kidney Int. 2022. PMID: 36041560 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

