Diabetes Technology Meeting 2021
- PMID: 35499170
- PMCID: PMC9264449
- DOI: 10.1177/19322968221090279
Diabetes Technology Meeting 2021
Abstract
Diabetes Technology Society hosted its annual Diabetes Technology Meeting on November 4 to November 6, 2021. This meeting brought together speakers to discuss various developments within the field of diabetes technology. Meeting topics included blood glucose monitoring, continuous glucose monitoring, novel sensors, direct-to-consumer telehealth, metrics for glycemia, software for diabetes, regulation of diabetes technology, diabetes data science, artificial pancreas, novel insulins, insulin delivery, skin trauma, metabesity, precision diabetes, diversity in diabetes technology, use of diabetes technology in pregnancy, and green diabetes. A live demonstration on a mobile app to monitor diabetic foot wounds was presented.
Keywords: diabetes; digital health; glucose; insulin; software; technology.
Conflict of interest statement
Figures






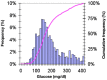


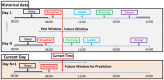




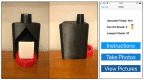
References
-
- Roche Diabetes Care. About iPDM—because there is no break from diabetes. Date unknown. https://www.rochediabetes.com/en-gb/about-ipdm. Accessed February 24, 2022.
-
- Accu-Chek®. Accu-Chek SugarView. Date unknown. https://www.accu-chek.com.pk/app-software/sugarview. Accessed February 24, 2022.
-
- Bauer S, Nauck MA. Polypharmacy in people with Type 1 and Type 2 diabetes is justified by current guidelines—a comprehensive assessment of drug prescriptions in patients needing inpatient treatment for diabetes-associated problems. Diabet Med J Br Diabet Assoc. 2014;31(9):1078-1085. doi:10.1111/dme.12497. - DOI - PubMed

