Indirect and Direct Effects of SARS-CoV-2 on Human Pancreatic Islets
- PMID: 35499468
- PMCID: PMC9490452
- DOI: 10.2337/db21-0926
Indirect and Direct Effects of SARS-CoV-2 on Human Pancreatic Islets
Abstract
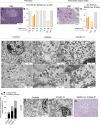
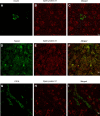
Recent studies have shown that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may induce metabolic distress, leading to hyperglycemia in patients affected by coronavirus disease 19 (COVID-19). We investigated the potential indirect and direct effects of SARS-CoV-2 on human pancreatic islets in 10 patients who became hyperglycemic after COVID-19. Although there was no evidence of peripheral anti-islet autoimmunity, the serum of these patients displayed toxicity on human pancreatic islets, which could be abrogated by the use of anti-interleukin-1β (IL-1β), anti-IL-6, and anti-tumor necrosis factor α, cytokines known to be highly upregulated during COVID-19. Interestingly, the receptors of those aforementioned cytokines were highly expressed on human pancreatic islets. An increase in peripheral unmethylated INS DNA, a marker of cell death, was evident in several patients with COVID-19. Pathology of the pancreas from deceased hyperglycemic patients who had COVID-19 revealed mild lymphocytic infiltration of pancreatic islets and pancreatic lymph nodes. Moreover, SARS-CoV-2-specific viral RNA, along with the presence of several immature insulin granules or proinsulin, was detected in postmortem pancreatic tissues, suggestive of β-cell-altered proinsulin processing, as well as β-cell degeneration and hyperstimulation. These data demonstrate that SARS-CoV-2 may negatively affect human pancreatic islet function and survival by creating inflammatory conditions, possibly with a direct tropism, which may in turn lead to metabolic abnormalities observed in patients with COVID-19.
Trial registration: ClinicalTrials.gov NCT04463849.
© 2022 by the American Diabetes Association.
Figures


References
-
- Yang JK, Feng Y, Yuan MY, et al. . Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet Med 2006;23:623–628 - PubMed

