Active Surveillance Program to Increase Awareness on Invasive Fungal Diseases: the French RESSIF Network (2012 to 2018)
- PMID: 35499498
- PMCID: PMC9239099
- DOI: 10.1128/mbio.00920-22
Active Surveillance Program to Increase Awareness on Invasive Fungal Diseases: the French RESSIF Network (2012 to 2018)
Abstract
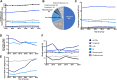
The French National Reference Center for Invasive Mycoses and Antifungals leads an active and sustained nationwide surveillance program on probable and proven invasive fungal diseases (IFDs) to determine their epidemiology in France. Between 2012 and 2018, a total of 10,886 IFDs were recorded. The incidence increased slightly over time (2.16 to 2.36/10,000 hospitalization days, P = 0.0562) in relation with an increase of fungemia incidence (1.03 to 1.19/10,000, P = 0.0023), while that of other IFDs remained stable. The proportion of ≥65-year-old patients increased from 38.4% to 45.3% (P < 0.0001). Yeast fungemia (n = 5,444) was due mainly to Candida albicans (55.6%) with stable proportions of species over time. Echinocandins became the main drug prescribed (46.7% to 61.8%), but global mortality rate remained unchanged (36.3% at 1 month). Pneumocystis jirovecii pneumonia (n = 2,106) was diagnosed mostly in HIV-negative patients (80.7%) with a significantly higher mortality than in HIV-positive patients (21.9% versus 5.4% at 1 month, P < 0.0001). Invasive aspergillosis (n = 1,661) and mucormycosis (n = 314) were diagnosed mostly in hematology (>60% of the cases) with a global mortality rate of 42.5% and 59.3%, respectively, at 3 months and significant changes in diagnosis procedure over time. More concurrent infections were also diagnosed over time (from 5.4% to 9.4% for mold IFDs, P = 0.0115). In conclusion, we observed an aging of patients with IFD with a significant increase in incidence only for yeast fungemia, a trend toward more concurrent infections, which raises diagnostic and therapeutic issues. Overall, global survival associated with IFDs has not improved despite updated guidelines and new diagnostic tools. IMPORTANCE The epidemiology of invasive fungal diseases (IFDs) is hard to delineate given the difficulties in ascertaining the diagnosis that is often based on the confrontation of clinical and microbiological criteria. The present report underlines the interest of active surveillance involving mycologists and clinicians to describe the global incidence and that of the main IFDs. Globally, although the incidence of Pneumocystis pneumonia, invasive aspergillosis, and mucormycosis remained stable over the study period (2012 to 2018), that of yeast fungemia increased slightly. We also show here that IFDs seem to affect older people more frequently. The most worrisome observation is the lack of improvement in the global survival rate associated with IFDs despite the increasing use of more sensitive diagnostic tools, the availability of new antifungal drugs very active in clinical trials, and a still low/marginal rate of acquired in vitro resistance in France. Therefore, other tracks of improvement should be investigated actively.
Keywords: aspergillosis; candidemia; epidemiology; invasive fungal infections; mucormycosis; pneumocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H, Alexander BD, Andes D, Azoulay É, Bialek R, Bradsher RW, Bretagne S, Calandra T, Caliendo AM, Castagnola E, Cruciani M, Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg B-J, Lagrou K, Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J, Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M, Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z, Roilides E, Ruhnke M, Prokop CS, Shoham S, et al. . 2020. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71:1367–1376. doi:10.1093/cid/ciz1008. - DOI - PMC - PubMed
-
- Andes DR, Safdar N, Baddley JW, Alexander B, Brumble L, Freifeld A, Hadley S, Herwaldt L, Kauffman C, Lyon GM, Morrison V, Patterson T, Perl T, Walker R, Hess T, Chiller T, Pappas PG, TRANSNET Investigators. 2016. The epidemiology and outcomes of invasive Candida infections among organ transplant recipients in the United States: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Transpl Infect Dis 18:921–931. doi:10.1111/tid.12613. - DOI - PubMed
-
- Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. 2011. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn Microbiol Infect Dis 69:45–50. doi:10.1016/j.diagmicrobio.2010.08.013. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

