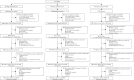
Evaluation of multiple micronutrient supplementation and medium-quantity lipid-based nutrient supplementation in pregnancy on child development in rural Niger: A secondary analysis of a cluster randomized controlled trial
- PMID: 35500028
- PMCID: PMC9060361
- DOI: 10.1371/journal.pmed.1003984
Evaluation of multiple micronutrient supplementation and medium-quantity lipid-based nutrient supplementation in pregnancy on child development in rural Niger: A secondary analysis of a cluster randomized controlled trial
Abstract
Background: It is estimated that over 250 million children under 5 years of age in low- and middle-income countries (LMICs) do not reach their full developmental potential. Poor maternal diet, anemia, and micronutrient deficiencies during pregnancy are associated with suboptimal neurodevelopmental outcomes in children. However, the effect of prenatal macronutrient and micronutrient supplementation on child development in LMIC settings remains unclear due to limited evidence from randomized trials.
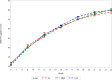
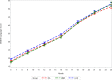
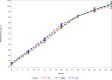
Methods and findings: We conducted a 3-arm cluster-randomized trial (n = 53 clusters) that evaluated the efficacy of (1) prenatal multiple micronutrient supplementation (MMS; n = 18 clusters) and (2) lipid-based nutrient supplementation (LNS; n = 18 clusters) as compared to (3) routine iron-folic acid (IFA) supplementation (n = 17 clusters) among pregnant women in the rural district of Madarounfa, Niger, from March 2015 to August 2019 (ClinicalTrials.gov identifier NCT02145000). Children were followed until 2 years of age, and the Bayley Scales of Infant and Toddler Development III (BSID-III) were administered to children every 3 months from 6 to 24 months of age. Maternal report of WHO gross motor milestone achievement was assessed monthly from 3 to 24 months of age. An intention-to-treat analysis was followed. Child BSID-III data were available for 559, 492, and 581 singleton children in the MMS, LNS, and IFA groups, respectively. Child WHO motor milestone data were available for 691, 781, and 753 singleton children in the MMS, LNS, and IFA groups, respectively. Prenatal MMS had no effect on child BSID-III cognitive (standardized mean difference [SMD]: 0.21; 95% CI: -0.20, 0.62; p = 0.32), language (SMD: 0.16; 95% CI: -0.30, 0.61; p = 0.50) or motor scores (SMD: 0.18; 95% CI: -0.39, 0.74; p = 0.54) or on time to achievement of the WHO gross motor milestones as compared to IFA. Prenatal LNS had no effect on child BSID-III cognitive (SMD: 0.17; 95% CI: -0.15, 0.49; p = 0.29), language (SMD: 0.11; 95% CI: -0.22, 0.44; p = 0.53) or motor scores (SMD: -0.04; 95% CI: -0.46, 0.37; p = 0.85) at the 24-month endline visit as compared to IFA. However, the trajectory of BSID-III cognitive scores during the first 2 years of life differed between the groups with children in the LNS group having higher cognitive scores at 18 and 21 months (approximately 0.35 SD) as compared to the IFA group (p-value for difference in trajectory <0.001). Children whose mothers received LNS also had earlier achievement of sitting alone (hazard ratio [HR]: 1.57; 95% CI: 1.10 to 2.24; p = 0.01) and walking alone (1.52; 95% CI: 1.14 to 2.03; p = 0.004) as compared to IFA, but there was no effect on time to achievement of other motor milestones. A limitation of our study is that we assessed child development up to 2 years of age, and, therefore, we may have not captured effects that are easier to detect or emerge at older ages.
Conclusions: There was no benefit of prenatal MMS on child development outcomes up to 2 years of age as compared to IFA. There was evidence of an apparent positive effect of prenatal LNS on cognitive development trajectory and time to achievement of selected gross motor milestones.
Trial registration: ClinicalTrials.gov NCT02145000.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: RFG is an Academic Editor on PLOS Medicine’s editorial board.
Figures