Unsupervised learning for robust working memory
- PMID: 35500033
- PMCID: PMC9098088
- DOI: 10.1371/journal.pcbi.1009083
Unsupervised learning for robust working memory
Abstract
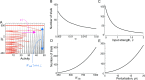
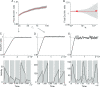
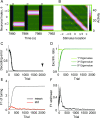
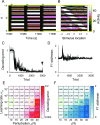
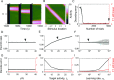
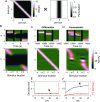
Working memory is a core component of critical cognitive functions such as planning and decision-making. Persistent activity that lasts long after the stimulus offset has been considered a neural substrate for working memory. Attractor dynamics based on network interactions can successfully reproduce such persistent activity. However, it requires a fine-tuning of network connectivity, in particular, to form continuous attractors which were suggested for encoding continuous signals in working memory. Here, we investigate whether a specific form of synaptic plasticity rules can mitigate such tuning problems in two representative working memory models, namely, rate-coded and location-coded persistent activity. We consider two prominent types of plasticity rules, differential plasticity correcting the rapid activity changes and homeostatic plasticity regularizing the long-term average of activity, both of which have been proposed to fine-tune the weights in an unsupervised manner. Consistent with the findings of previous works, differential plasticity alone was enough to recover a graded-level persistent activity after perturbations in the connectivity. For the location-coded memory, differential plasticity could also recover persistent activity. However, its pattern can be irregular for different stimulus locations under slow learning speed or large perturbation in the connectivity. On the other hand, homeostatic plasticity shows a robust recovery of smooth spatial patterns under particular types of synaptic perturbations, such as perturbations in incoming synapses onto the entire or local populations. However, homeostatic plasticity was not effective against perturbations in outgoing synapses from local populations. Instead, combining it with differential plasticity recovers location-coded persistent activity for a broader range of perturbations, suggesting compensation between two plasticity rules.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Goldman MS, Compte A, Wang XJ. Neural Integrator Models. Encyclopedia of Neuroscience. Elsevier Ltd; 2009. pp. 165–178. doi: 10.1016/B978-008045046-9.01434–0 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

