Spectroscopic Fingerprints of Cavity Formation and Solute Insertion as a Measure of Hydration Entropic Loss and Enthalpic Gain
- PMID: 35500074
- PMCID: PMC9401576
- DOI: 10.1002/anie.202203893
Spectroscopic Fingerprints of Cavity Formation and Solute Insertion as a Measure of Hydration Entropic Loss and Enthalpic Gain
Abstract
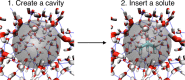
Hydration free energies are dictated by a subtle balance of hydrophobic and hydrophilic interactions. We present here a spectroscopic approach, which gives direct access to the two main contributions: Using THz-spectroscopy to probe the frequency range of the intermolecular stretch (150-200 cm-1 ) and the hindered rotations (450-600 cm-1 ), the local contributions due to cavity formation and hydrophilic interactions can be traced back. We show that via THz calorimetry these fingerprints can be correlated 1 : 1 with the group specific solvation entropy and enthalpy. This allows to deduce separately the hydrophobic (i.e. cavity formation) and hydrophilic contributions to thermodynamics, as shown for hydrated alcohols as a case study. Accompanying molecular dynamics simulations quantitatively support our experimental results. In the future our approach will allow to dissect hydration contributions in inhomogeneous mixtures and under non-equilibrium conditions.
Keywords: Alcohol Hydration; Enthalpy; Entropy; Hydrophobic Hydration; THz Spectroscopy; THz-Calorimetry.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

