Structure and role of the linker domain of the iron surface-determinant protein IsdH in heme transportation in Staphylococcus aureus
- PMID: 35500652
- PMCID: PMC9163592
- DOI: 10.1016/j.jbc.2022.101995
Structure and role of the linker domain of the iron surface-determinant protein IsdH in heme transportation in Staphylococcus aureus
Abstract
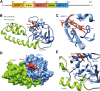
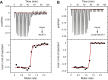
Staphylococcus aureus is a major cause of deadly nosocomial infections, a severe problem fueled by the steady increase of resistant bacteria. The iron surface determinant (Isd) system is a family of proteins that acquire nutritional iron from the host organism, helping the bacterium to proliferate during infection, and therefore represents a promising antibacterial target. In particular, the surface protein IsdH captures hemoglobin (Hb) and acquires the heme moiety containing the iron atom. Structurally, IsdH comprises three distinctive NEAr-iron Transporter (NEAT) domains connected by linker domains. The objective of this study was to characterize the linker region between NEAT2 and NEAT3 from various biophysical viewpoints and thereby advance our understanding of its role in the molecular mechanism of heme extraction. We demonstrate the linker region contributes to the stability of the bound protein, likely influencing the flexibility and orientation of the NEAT3 domain in its interaction with Hb, but only exerts a modest contribution to the affinity of IsdH for heme. Based on these data, we suggest that the flexible nature of the linker facilitates the precise positioning of NEAT3 to acquire heme. In addition, we also found that residues His45 and His89 of Hb located in the heme transfer route toward IsdH do not play a critical role in the transfer rate-determining step. In conclusion, this study clarifies key elements of the mechanism of heme extraction of human Hb by IsdH, providing key insights into the Isd system and other protein systems containing NEAT domains.
Keywords: Staphylococcus aureus; heme; heme acquisition; heme transport; hemoglobin; hemoglobin receptor; hydrogen exchange mass spectrometry; iron surface determinant system; structure-function; x-ray crystallography.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interests The authors declare no conflict of interest.
Figures






References
-
- Diekema D.J., Pfaller M.A., Schmitz F.J., Smayevsky J., Bell J., Jones R.N., et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 2001;32:S114–S132. - PubMed
-
- Brennan G.I., Abbott Y., Burns A., Leonard F., McManus B.A., O'Connell B., et al. The emergence and spread of multiple livestock-associated clonal complex 398 methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains among animals and humans in the republic of Ireland, 2010-2014. PLoS One. 2016;11 - PMC - PubMed
-
- Dryla A., Gelbmann D., von Gabain A., Nagy E. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol. Microbiol. 2003;49:37–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

