Multimodality imaging of nanoparticle-based vaccines: Shedding light on immunology
- PMID: 35501142
- PMCID: PMC9481661
- DOI: 10.1002/wnan.1807
Multimodality imaging of nanoparticle-based vaccines: Shedding light on immunology
Abstract
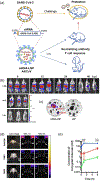
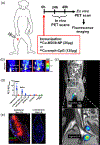
In recent years, there have been significant innovations in the development of nanoparticle-based vaccines and vaccine delivery systems. For the purposes of both design and evaluation, these nanovaccines are imaged using the wealth of understanding established around medical imaging of nanomaterials. An important insight to the advancement of the field of nanovaccines can be given by an analysis of the design rationale of an imaging platform, as well as the significance of the information provided by imaging. Nanovaccine imaging strategies can be categorized by the imaging modality leveraged, but it is also worth understanding the superiority or convenience of a given modality over others in a given context of a particular nanovaccine. The most important imaging modalities in this endeavor are optical imaging including near-infrared fluorescence imaging (NIRF), emission tomography methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT) with or without computed tomography (CT) or magnetic resonance (MR), the emerging magnetic particle imaging (MPI), and finally, multimodal applications of imaging which include molecular imaging with magnetic resonance imaging (MRI) and photoacoustic (PA) imaging. One finds that each of these modalities has strengths and weaknesses, but optical and PET imaging tend, in this context, to be currently the most accessible, convenient, and informative modalities. Nevertheless, an important principle is that there is not a one-size-fits-all solution, and that the specific nanovaccine in question must be compatible with a particular imaging modality. This article is categorized under: Nanotechnology Approaches to Biology > Nanoscale Systems in Biology Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease Therapeutic Approaches and Drug Discovery > Nanomedicine for Infectious Disease.
Keywords: fluorescence imaging; medical imaging; nanomaterials; positron emission tomography; vaccines.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
Weibo Cai is a scientific advisor, stockholder, and grantee of Focus-X Therapeutics, Inc. All other authors declare that they have no conflict of interest.
Figures




References
-
- Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, & Krummel MF (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine, 24(5), 541–550. 10.1038/s41591-018-0014-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

