Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: Interim report of a phase I/II randomized controlled trial
- PMID: 35501178
- PMCID: PMC9053833
- DOI: 10.1016/j.vaccine.2022.04.035
Safety and immunogenicity of NVX-CoV2373 (TAK-019) vaccine in healthy Japanese adults: Interim report of a phase I/II randomized controlled trial
Abstract
Background: We evaluated the safety and immunogenicity of NVX-CoV2373, a recombinant SARS-CoV-2 nanoparticle vaccine, in healthy Japanese participants.
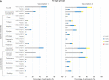
Methods: This phase 1/2, randomized, observer-blind, placebo-controlled trial conducted in Japan (two sites), enrolled healthy Japanese adults aged ≥ 20 years with no history/risk of SARS-CoV-2 infection and no prior exposure to other approved/investigational SARS-CoV-2 vaccines or treatments. Participants were stratified by age (< 65 or ≥ 65 years) and randomized to receive two doses of either NVX-CoV2373 (5 μg SARS-CoV-2 rS; 50 μg Matrix-M1) or placebo, 21 days apart. Primary outcomes were safety and immunogenicity assessed by serum IgG antibody levels against SARS-CoV-2 rS protein on day 36. Herein, we report the primary data analysis at 4 weeks after the second dose, ahead of 12-month follow-up completion (data cut-off: 8 May 2021).
Results: Between 12 February 2021 and 17 March 2021, 326 subjects were screened, and 200 participants enrolled and randomized: NVX-CoV2373, n = 150; placebo, n = 50. Solicited adverse events (AEs) through 7 days after each injection occurred in 121/150 (80.7%) and 11/50 (22.0%) participants in the NVX-CoV2373 and placebo arms, respectively. In the NVX-CoV2373 arm, tenderness and injection site pain were the most frequently reported solicited AEs after each vaccination, irrespective of age. Robust immune responses occurred with NVX-CoV2373 (n = 150) by day 36: IgG geometric mean fold rise (95% confidence interval) 259 (219, 306); seroconversion rate 100% (97.6, 100). No such response occurred with placebo (n = 49).
Conclusion: Two doses of NVX-CoV2373 given with a 21-day interval demonstrated acceptable safety and induced robust anti-SARS-CoV-2 immune responses in healthy Japanese adults.
Funding: Takeda Pharmaceutical Company Limited and Japan Agency for Medical Research and Development (AMED).
Clinicaltrials: gov identifier: NCT04712110.
Keywords: COVID-19; Immunogenicity; Japanese adults; NVX-CoV2373; SARS-CoV-2; Safety.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest TM, KM, KS, SS, and MM are employees of Takeda Pharmaceutical Company Ltd. RPS is an employee of Takeda Pharmaceuticals International AG. KM holds stocks in Takeda Pharmaceutical Company Ltd.
Figures



References
-
- Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

