Protein-Protein Recognition Involved in the Intermodular Transacylation Reaction in Modular Polyketide Synthase in the Biosynthesis of Vicenistatin
- PMID: 35501288
- PMCID: PMC9401018
- DOI: 10.1002/cbic.202200200
Protein-Protein Recognition Involved in the Intermodular Transacylation Reaction in Modular Polyketide Synthase in the Biosynthesis of Vicenistatin
Abstract
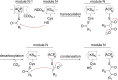
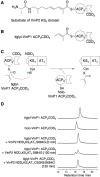
The ketosynthase (KS) domain is a core domain found in modular polyketide synthases (PKSs). To maintain the polyketide biosynthetic fidelity, the KS domain must only accept an acyl group from the acyl carrier protein (ACP) domain of the immediate upstream module even when they are separated into different polypeptides. Although it was reported that both the docking domain-based interactions and KS-ACP compatibility are important for the interpolypeptide transacylation reaction in 6-deoxyerythronolide B synthase, it is not clear whether these findings are broadly applied to other modular PKSs. Herein, we describe the importance of protein-protein recognition in the intermodular transacylation between VinP1 module 3 and VinP2 module 4 in vicenistatin biosynthesis. We compared the transacylation activity and crosslinking efficiency of VinP2 KS4 against the cognate VinP1 ACP3 with the noncognate one. As a result, it appeared that VinP2 KS4 distinguishes the cognate ACP3 from other ACPs.
Keywords: acyl carrier protein; biosynthesis; ketosynthases; polyketide synthases; protein-protein interactions.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

