Maladaptive myelination promotes generalized epilepsy progression
- PMID: 35501379
- PMCID: PMC9076538
- DOI: 10.1038/s41593-022-01052-2
Maladaptive myelination promotes generalized epilepsy progression
Abstract
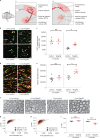
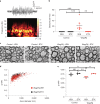
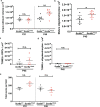
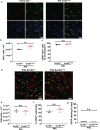
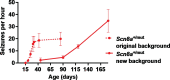
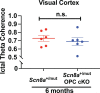
Activity-dependent myelination can fine-tune neural network dynamics. Conversely, aberrant neuronal activity, as occurs in disorders of recurrent seizures (epilepsy), could promote maladaptive myelination, contributing to pathogenesis. In this study, we tested the hypothesis that activity-dependent myelination resulting from absence seizures, which manifest as frequent behavioral arrests with generalized electroencephalography (EEG) spike-wave discharges, promote thalamocortical network hypersynchrony and contribute to epilepsy progression. We found increased oligodendrogenesis and myelination specifically within the seizure network in two models of generalized epilepsy with absence seizures (Wag/Rij rats and Scn8a+/mut mice), evident only after epilepsy onset. Aberrant myelination was prevented by pharmacological seizure inhibition in Wag/Rij rats. Blocking activity-dependent myelination decreased seizure burden over time and reduced ictal synchrony as assessed by EEG coherence. These findings indicate that activity-dependent myelination driven by absence seizures contributes to epilepsy progression; maladaptive myelination may be pathogenic in some forms of epilepsy and other neurological diseases.
© 2022. The Author(s).
Conflict of interest statement
M.M. is on the Scientific Advisory Board of Cygnal Therapeutics. The authors declare no other competing interests.
Figures








Comment in
-
Seizures beget seizures.Nat Rev Neurosci. 2022 Jul;23(7):392-393. doi: 10.1038/s41583-022-00604-6. Nat Rev Neurosci. 2022. PMID: 35606498 No abstract available.
-
That's a Wrap: Could Controlling Activity-Regulated Myelination Prevent Absence Seizures?Epilepsy Curr. 2022 Sep 22;22(6):395-397. doi: 10.1177/15357597221123898. eCollection 2022 Nov-Dec. Epilepsy Curr. 2022. PMID: 36426188 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

