Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis
- PMID: 35501382
- PMCID: PMC9081225
- DOI: 10.1038/s41593-022-01060-2
Cerebrospinal fluid can exit into the skull bone marrow and instruct cranial hematopoiesis in mice with bacterial meningitis
Abstract
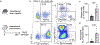
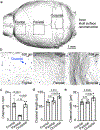
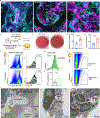
Interactions between the immune and central nervous systems strongly influence brain health. Although the blood-brain barrier restricts this crosstalk, we now know that meningeal gateways through brain border tissues facilitate intersystem communication. Cerebrospinal fluid (CSF), which interfaces with the glymphatic system and thereby drains the brain's interstitial and perivascular spaces, facilitates outward signaling beyond the blood-brain barrier. In the present study, we report that CSF can exit into the skull bone marrow. Fluorescent tracers injected into the cisterna magna of mice migrate along perivascular spaces of dural blood vessels and then travel through hundreds of sub-millimeter skull channels into the calvarial marrow. During meningitis, bacteria hijack this route to invade the skull's hematopoietic niches and initiate cranial hematopoiesis ahead of remote tibial sites. As skull channels also directly provide leukocytes to meninges, the privileged sampling of brain-derived danger signals in CSF by regional marrow may have broad implications for inflammatory neurological disorders.
© 2022. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



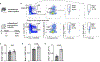








Comment in
-
How the brain regulates its own immune system.Nat Neurosci. 2022 May;25(5):532-534. doi: 10.1038/s41593-022-01066-w. Nat Neurosci. 2022. PMID: 35501381 No abstract available.
-
Immune gains through brain drains.Sci Immunol. 2022 Jun 3;7(72):eadd2045. doi: 10.1126/sciimmunol.add2045. Epub 2022 Jun 3. Sci Immunol. 2022. PMID: 35658011
References
-
- Ransohoff RM, Kivisakk P & Kidd G Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol 3, 569–581 (2003). - PubMed
-
- Engelhardt B, Vajkoczy P & Weller RO The movers and shapers in immune privilege of the CNS. Nat Immunol 18, 123–131 (2017). - PubMed
-
- Gres V, Kolter J, Erny D & Henneke P The role of CNS macrophages in streptococcal meningoencephalitis. J Leukoc Biol 106, 209–218 (2019). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

