Lymphocyte activation gene-3-associated protein networks are associated with HDL-cholesterol and mortality in the Trans-omics for Precision Medicine program
- PMID: 35501457
- PMCID: PMC9061762
- DOI: 10.1038/s42003-022-03304-0
Lymphocyte activation gene-3-associated protein networks are associated with HDL-cholesterol and mortality in the Trans-omics for Precision Medicine program
Abstract
Deficiency of the immune checkpoint lymphocyte activation gene-3 (LAG3) protein is significantly associated with both elevated HDL-cholesterol (HDL-C) and myocardial infarction risk. We determined the association of genetic variants within ±500 kb of LAG3 with plasma LAG3 and defined LAG3-associated plasma proteins with HDL-C and clinical outcomes. Whole genome sequencing and plasma proteomics were obtained from the Multi-Ethnic Study of Atherosclerosis (MESA) and the Framingham Heart Study (FHS) cohorts as part of the Trans-Omics for Precision Medicine program. In situ Hi-C chromatin capture was performed in EBV-transformed cell lines isolated from four MESA participants. Genetic association analyses were performed in MESA using multivariate regression models, with validation in FHS. A LAG3-associated protein network was tested for association with HDL-C, coronary heart disease, and all-cause mortality. We identify an association between the LAG3 rs3782735 variant and plasma LAG3 protein. Proteomics analysis reveals 183 proteins significantly associated with LAG3 with four proteins associated with HDL-C. Four proteins discovered for association with all-cause mortality in FHS shows nominal associations in MESA. Chromatin capture analysis reveals significant cis interactions between LAG3 and C1S, LRIG3, TNFRSF1A, and trans interactions between LAG3 and B2M. A LAG3-associated protein network has significant associations with HDL-C and mortality.
© 2022. The Author(s).
Conflict of interest statement
A.R. reports inventorship rights to patents held by University of Connecticut Health and Lipid Genomics. A.R. is the founder of Lipid Genomics. The remaining authors declare no competing interests.
Figures

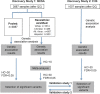
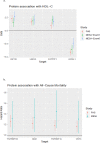

Similar articles
-
Lymphocyte activation gene 3 and coronary artery disease.JCI Insight. 2016 Oct 20;1(17):e88628. doi: 10.1172/jci.insight.88628. JCI Insight. 2016. PMID: 27777974 Free PMC article.
-
Soluble Immune Checkpoint Protein and Lipid Network Associations with All-Cause Mortality Risk: Trans-Omics for Precision Medicine (TOPMed) Program.medRxiv [Preprint]. 2025 Jan 9:2025.01.08.25320225. doi: 10.1101/2025.01.08.25320225. medRxiv. 2025. PMID: 39830278 Free PMC article. Preprint.
-
High HDL-Cholesterol Paradox: SCARB1-LAG3-HDL Axis.Curr Atheroscler Rep. 2021 Jan 5;23(1):5. doi: 10.1007/s11883-020-00902-3. Curr Atheroscler Rep. 2021. PMID: 33398433 Free PMC article. Review.
-
Visit-to-visit Lipid Variability, Coronary Artery Calcification, Inflammation, and Mortality in the Multi-Ethnic Study of Atherosclerosis (MESA).Eur J Prev Cardiol. 2025 Feb 20:zwaf085. doi: 10.1093/eurjpc/zwaf085. Online ahead of print. Eur J Prev Cardiol. 2025. PMID: 39977247
-
HDL Cholesterol Metabolism and the Risk of CHD: New Insights from Human Genetics.Curr Cardiol Rep. 2017 Nov 4;19(12):132. doi: 10.1007/s11886-017-0940-0. Curr Cardiol Rep. 2017. PMID: 29103089 Review.
Cited by
-
Fibrinogen-Like Protein 1 as a Novel Biomarker of Psoriasis Severity.J Inflamm Res. 2022 Aug 15;15:4637-4647. doi: 10.2147/JIR.S378953. eCollection 2022. J Inflamm Res. 2022. PMID: 35996685 Free PMC article.
-
Higher HDL cholesterol levels are associated with increased markers of interstitial myocardial fibrosis in the MultiEthnic Study of Atherosclerosis (MESA).Sci Rep. 2023 Nov 17;13(1):20115. doi: 10.1038/s41598-023-46811-8. Sci Rep. 2023. PMID: 37978334 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR033176/RR/NCRR NIH HHS/United States
- U54 HG003067/HG/NHGRI NIH HHS/United States
- R01 HL071259/HL/NHLBI NIH HHS/United States
- R01 HL092577/HL/NHLBI NIH HHS/United States
- 75N92020D00001/HL/NHLBI NIH HHS/United States
- R01 HL071250/HL/NHLBI NIH HHS/United States
- N01 HC095167/HL/NHLBI NIH HHS/United States
- R01 HL105756/HL/NHLBI NIH HHS/United States
- HHSN268201500001I/HL/NHLBI NIH HHS/United States
- R01 HL071205/HL/NHLBI NIH HHS/United States
- N01 HC095166/HL/NHLBI NIH HHS/United States
- R01 HL071258/HL/NHLBI NIH HHS/United States
- N01 HC095160/HL/NHLBI NIH HHS/United States
- 75N92020D00002/HL/NHLBI NIH HHS/United States
- HHSN268201500003C/HL/NHLBI NIH HHS/United States
- P30 DK040561/DK/NIDDK NIH HHS/United States
- N01 HC095161/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- N01 HC095168/HL/NHLBI NIH HHS/United States
- R01 HL071251/HL/NHLBI NIH HHS/United States
- R01 HL120393/HL/NHLBI NIH HHS/United States
- HHSN268201500001C/HL/NHLBI NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01 HC095169/HL/NHLBI NIH HHS/United States
- R01 HL131862/HL/NHLBI NIH HHS/United States
- N01 HC095159/HL/NHLBI NIH HHS/United States
- 75N92020D00003/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- R01 HL071051/HL/NHLBI NIH HHS/United States
- HHSN268201800001C/HL/NHLBI NIH HHS/United States
- N01 HC025195/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- 75N92020D00004/HL/NHLBI NIH HHS/United States
- N01 HC095163/HL/NHLBI NIH HHS/United States
- 75N92020D00007/HL/NHLBI NIH HHS/United States
- HHSN268201500014C/HL/NHLBI NIH HHS/United States
- HHSN268201500003I/HL/NHLBI NIH HHS/United States
- 75N92019D00031/HL/NHLBI NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- 75N92020D00006/HL/NHLBI NIH HHS/United States
- R01 HL117626/HL/NHLBI NIH HHS/United States
- N01 HC095162/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- N01 HC095165/HL/NHLBI NIH HHS/United States
- N01 HC095164/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

