Sugars dominate the seagrass rhizosphere
- PMID: 35501482
- PMCID: PMC9262712
- DOI: 10.1038/s41559-022-01740-z
Sugars dominate the seagrass rhizosphere
Abstract
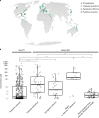
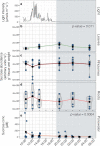
Seagrasses are among the most efficient sinks of carbon dioxide on Earth. While carbon sequestration in terrestrial plants is linked to the microorganisms living in their soils, the interactions of seagrasses with their rhizospheres are poorly understood. Here, we show that the seagrass, Posidonia oceanica excretes sugars, mainly sucrose, into its rhizosphere. These sugars accumulate to µM concentrations-nearly 80 times higher than previously observed in marine environments. This finding is unexpected as sugars are readily consumed by microorganisms. Our experiments indicated that under low oxygen conditions, phenolic compounds from P. oceanica inhibited microbial consumption of sucrose. Analyses of the rhizosphere community revealed that many microbes had the genes for degrading sucrose but these were only expressed by a few taxa that also expressed genes for degrading phenolics. Given that we observed high sucrose concentrations underneath three other species of marine plants, we predict that the presence of plant-produced phenolics under low oxygen conditions allows the accumulation of labile molecules across aquatic rhizospheres.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










Comment in
-
A sweet spot in marine ecosystems.Nat Ecol Evol. 2022 Jul;6(7):847-848. doi: 10.1038/s41559-022-01764-5. Nat Ecol Evol. 2022. PMID: 35501481 No abstract available.
References
-
- Short F, Carruthers T, Dennison W, Waycott M. Global seagrass distribution and diversity: a bioregional model. J. Exp. Mar. Biol. Ecol. 2007;350:3–20.
-
- Barbier E, et al. The value of estuarine and coastal ecosystem services. Ecol. Monogr. 2011;81:169–193.
-
- Fourqurean JW, et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 2012;5:505–509.
-
- McLeod E, et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011;9:552–560.
-
- Barrón C, Duarte CM. Dissolved organic matter release in a Posidonia oceanica meadow. Marine Ecol. Prog. Ser. 2009;374:75–84.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

