Impact of COVID-19 vaccine on epilepsy in adult subjects: an Italian multicentric experience
- PMID: 35501537
- PMCID: PMC9059692
- DOI: 10.1007/s10072-022-06100-0
Impact of COVID-19 vaccine on epilepsy in adult subjects: an Italian multicentric experience
Abstract
Objectives: To investigate the safety and tolerability of COVID-19 vaccines in people with epilepsy (PwE).
Methods: In this multicentric observational cohort study, we recruited adult patients (age > 18 years old) with epilepsy who attended the Outpatient Epilepsy Clinic from 1st July to 30th October 2021. We administered to the patients a structured questionnaire and interview on demographic and epilepsy characteristics, current treatment, previous SARS-CoV-2 infection, vaccine characteristics, post-vaccine seizure relapse, other side effect, variation of sleep habits, caffeine, or alcohol intake. Seizure frequency worsening was defined as a ratio between mean monthly frequency post-vaccination and mean monthly frequency pre-vaccination superior to 1. Patients were categorized in two groups: patients with seizure frequency worsening (WORSE) and patients with seizure stability (STABLE).
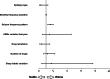
Results: A total of 358 people participated with a mean age of 47.46 ± 19.04. Focal seizure (79.1%), generalized epilepsy (20.4%), and unknown types of epilepsy (0.5%) were detected among participants. In total, 31 (8.7%) people expressed that they were not willing to receive a COVID-19 vaccine; 302 patients (92.35%) did not experience an increase in the seizure frequency (STABLE-group) whereas 25 patients (7.65%) had a seizure worsening (WORSE-group). Post-vaccine seizures occurred mainly in the 7 days following the administration of the vaccine. Patients in the WORSE-group were treated with a mean higher number of anti-seizure medication (ASMs) (p = 0.003) and had a higher pre-vaccine seizure frequency (p = 0.009) compared with patients in the STABLE-group. Drug-resistant epilepsy was also associated with seizure worsening (p = 0.01). One-year pre-vaccination seizure frequency pattern demonstrated that patients in the WORSE-group had a higher frequency pattern (p < 0.001). Multivariate analysis of the vaccinated group showed that only the seizure frequency pattern (confidence interval [CI] = 1.257-2.028; p < 0.001) was significantly associated with seizure worsening.
Conclusion: In our cohort of vaccinated PwE, only a little percentage had a transient short-term increase of seizure frequency. The present study demonstrates that COVID-19 vaccines have a good safety and tolerability profile in the short term in PwE.
Keywords: COVID-19; Coronavirus; Epilepsy; Seizures; Vaccine.
© 2022. The Author(s).
Conflict of interest statement
None.
Figures
Comment in
-
Safety of COVID-19 vaccine in patients with epilepsy: a meta-analysis.Neurol Sci. 2023 Jan;44(1):13-17. doi: 10.1007/s10072-022-06453-6. Epub 2022 Oct 15. Neurol Sci. 2023. PMID: 36243893 Free PMC article. No abstract available.
References
-
- WHO, Draft landscape of COVID-19 candidate vaccines, World Health Organisation (2020).
-
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. - DOI - PMC - PubMed
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC. Gruber, Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, Dold C, Emary KRW, Fedosyuk S, Fuskova M, Gbesemete D, Green C, Hallis B, Hou MM, Jenkin D, Joe CCD, Kelly EJ, Kerridge S, Lawrie AM, Lelliott A, Lwin MN, Makinson R, Marchevsky NG, Mujadidi Y, Munro APS, Pacurar M, Plested E, Rand J, Rawlinson T, Rhead S, Robinson H, Ritchie AJ, Ross-Russell AL, Saich S, Singh N, Smith CC, Snape MD, Song R, Tarrant R, Themistocleous Y, Thomas KM, Villafana TL, Warren SC, Watson MEE, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Faust SN, Pollard AJ. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous