Comparative effectiveness of smartphone healthcare applications for improving quality of life in lung cancer patients: study protocol
- PMID: 35501757
- PMCID: PMC9063346
- DOI: 10.1186/s12890-022-01970-8
Comparative effectiveness of smartphone healthcare applications for improving quality of life in lung cancer patients: study protocol
Abstract
Background: Although pulmonary rehabilitation is helpful for patients following lung cancer surgery, rehabilitation is not widely available, due in part to a lack of medical resources. Recent developments in digital health care have overcome the space limitations associated with in-person health care. This study will evaluate and compare the efficacy of three different smartphone healthcare systems in patients with lung cancer.
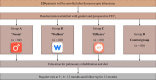
Methods: This single center randomized controlled study is designed to evaluate the efficacy of digital healthcare applications for lung cancer patients after thoracoscopic lung resection. A total of 320 patients will be enrolled and randomized 1:1:1:1 into four different groups, with one group each using the smartphone applications NOOM, Walkon, and Efilcare and the fourth being the control group without intervention. Questionnaires will be administered to patients at baseline and after 3, 6, and 12 months. The primary endpoint will be the score on the EuroQol five-dimension index. Secondary endpoints will include other questionnaires about quality of life and dyspnea.
Discussion: This prospective randomized controlled study may allow assessments and comparisons of the efficacy of various smartphone applications in patients who undergo lung cancer surgery. This process may enable the introduction of healthcare interventions that maintain quality of life in patients with lung cancer. Trial registration CRIS, KCT0005447. Registered 06 October 2020, https://cris.nih.go.kr/cris/search/detailSearch.do/19346.
Keywords: Exercise; Lung cancer; Pulmonary rehabilitation; Quality of life; Smartphone application.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical