A deep learning approach identifies new ECG features in congenital long QT syndrome
- PMID: 35501785
- PMCID: PMC9063181
- DOI: 10.1186/s12916-022-02350-z
A deep learning approach identifies new ECG features in congenital long QT syndrome
Abstract
Background: Congenital long QT syndrome (LQTS) is a rare heart disease caused by various underlying mutations. Most general cardiologists do not routinely see patients with congenital LQTS and may not always recognize the accompanying ECG features. In addition, a proportion of disease carriers do not display obvious abnormalities on their ECG. Combined, this can cause underdiagnosing of this potentially life-threatening disease.
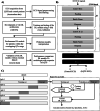
Methods: This study presents 1D convolutional neural network models trained to identify genotype positive LQTS patients from electrocardiogram as input. The deep learning (DL) models were trained with a large 10-s 12-lead ECGs dataset provided by Amsterdam UMC and externally validated with a dataset provided by University Hospital Leuven. The Amsterdam dataset included ECGs from 10000 controls, 172 LQTS1, 214 LQTS2, and 72 LQTS3 patients. The Leuven dataset included ECGs from 2200 controls, 32 LQTS1, and 80 LQTS2 patients. The performance of the DL models was compared with conventional QTc measurement and with that of an international expert in congenital LQTS (A.A.M.W). Lastly, an explainable artificial intelligence (AI) technique was used to better understand the prediction models.
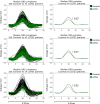
Results: Overall, the best performing DL models, across 5-fold cross-validation, achieved on average a sensitivity of 84 ± 2%, 90 ± 2% and 87 ± 6%, specificity of 96 ± 2%, 95 ± 1%, and 92 ± 4%, and AUC of 0.90 ± 0.01, 0.92 ± 0.02, and 0.89 ± 0.03, for LQTS 1, 2, and 3 respectively. The DL models were also shown to perform better than conventional QTc measurements in detecting LQTS patients. Furthermore, the performances held up when the DL models were validated on a novel external cohort and outperformed the expert cardiologist in terms of specificity, while in terms of sensitivity, the DL models and the expert cardiologist in LQTS performed the same. Finally, the explainable AI technique identified the onset of the QRS complex as the most informative region to classify LQTS from non-LQTS patients, a feature previously not associated with this disease.
Conclusions: This study suggests that DL models can potentially be used to aid cardiologists in diagnosing LQTS. Furthermore, explainable DL models can be used to possibly identify new features for LQTS on the ECG, thus increasing our understanding of this syndrome.
Keywords: Deep learning; ECG; Explainable AI; LQTS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Use of Artificial Intelligence and Deep Neural Networks in Evaluation of Patients With Electrocardiographically Concealed Long QT Syndrome From the Surface 12-Lead Electrocardiogram.JAMA Cardiol. 2021 May 1;6(5):532-538. doi: 10.1001/jamacardio.2020.7422. JAMA Cardiol. 2021. PMID: 33566059 Free PMC article.
-
Deep Learning-Augmented ECG Analysis for Screening and Genotype Prediction of Congenital Long QT Syndrome.JAMA Cardiol. 2024 Apr 1;9(4):377-384. doi: 10.1001/jamacardio.2024.0039. JAMA Cardiol. 2024. PMID: 38446445 Free PMC article.
-
Deep Neural Network Analysis of the 12-Lead Electrocardiogram Distinguishes Patients With Congenital Long QT Syndrome From Patients With Acquired QT Prolongation.Mayo Clin Proc. 2025 Feb;100(2):276-289. doi: 10.1016/j.mayocp.2024.07.016. Epub 2025 Jan 11. Mayo Clin Proc. 2025. PMID: 39797862
-
Genotype-specific ECG patterns in long QT syndrome.J Electrocardiol. 2006 Oct;39(4 Suppl):S101-6. doi: 10.1016/j.jelectrocard.2006.05.017. Epub 2006 Sep 11. J Electrocardiol. 2006. PMID: 16963070 Review.
-
Long QT syndrome and short QT syndrome.Prog Cardiovasc Dis. 2008 Nov-Dec;51(3):264-78. doi: 10.1016/j.pcad.2008.10.006. Prog Cardiovasc Dis. 2008. PMID: 19026859 Review.
Cited by
-
Multimodal fusion learning for long QT syndrome pathogenic genotypes in a racially diverse population.NPJ Digit Med. 2024 Aug 24;7(1):226. doi: 10.1038/s41746-024-01218-1. NPJ Digit Med. 2024. PMID: 39181999 Free PMC article.
-
A review of evaluation approaches for explainable AI with applications in cardiology.Artif Intell Rev. 2024;57(9):240. doi: 10.1007/s10462-024-10852-w. Epub 2024 Aug 9. Artif Intell Rev. 2024. PMID: 39132011 Free PMC article.
-
Mutation-Specific Differences in Kv7.1 (KCNQ1) and Kv11.1 (KCNH2) Channel Dysfunction and Long QT Syndrome Phenotypes.Int J Mol Sci. 2022 Jul 2;23(13):7389. doi: 10.3390/ijms23137389. Int J Mol Sci. 2022. PMID: 35806392 Free PMC article. Review.
-
Artificial Intelligence in Diagnosis of Long QT Syndrome: A Review of Current State, Challenges, and Future Perspectives.Mayo Clin Proc Digit Health. 2023 Dec 18;2(1):21-31. doi: 10.1016/j.mcpdig.2023.11.003. eCollection 2024 Mar. Mayo Clin Proc Digit Health. 2023. PMID: 40206686 Free PMC article. Review.
-
Clinical Applications, Methodology, and Scientific Reporting of Electrocardiogram Deep-Learning Models: A Systematic Review.JACC Adv. 2023 Dec;2(10):100686. doi: 10.1016/j.jacadv.2023.100686. Epub 2023 Nov 8. JACC Adv. 2023. PMID: 38288263 Free PMC article.
References
-
- Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. - DOI - PubMed
-
- Bazett HC. An analysis of the time-relations of electrocardiograms. Ann Noninvasive Electrocardiol. 1997;2:177–194. doi: 10.1111/j.1542-474X.1997.tb00325.x. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

