Stanniocalcin 2 (STC2): a universal tumour biomarker and a potential therapeutical target
- PMID: 35501821
- PMCID: PMC9063168
- DOI: 10.1186/s13046-022-02370-w
Stanniocalcin 2 (STC2): a universal tumour biomarker and a potential therapeutical target
Abstract
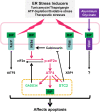
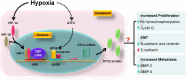
Stanniocalcin 2 (STC2) is a glycoprotein which is expressed in a broad spectrum of tumour cells and tumour tissues derived from human breast, colorectum, stomach, esophagus, prostate, kidney, liver, bone, ovary, lung and so forth. The expression of STC2 is regulated at both transcriptional and post-transcriptional levels; particularly, STC2 is significantly stimulated under various stress conditions like ER stress, hypoxia and nutrient deprivation. Biologically, STC2 facilitates cells dealing with stress conditions and prevents apoptosis. Importantly, STC2 also promotes the development of acquired resistance to chemo- and radio- therapies. In addition, multiple groups have reported that STC2 overexpression promotes cell proliferation, migration and immune response. Therefore, the overexpression of STC2 is positively correlated with tumour growth, invasion, metastasis and patients' prognosis, highlighting its potential as a biomarker and a therapeutic target. This review focuses on discussing the regulation, biological functions and clinical importance of STC2 in human cancers. Future perspectives in this field will also be discussed.
Keywords: Cancer therapy; Prognosis; Stanniocalcin 2; Stress Response; Tumorigenesis; Tumour progression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Stanniocalcin-2 (STC2): A potential lung cancer biomarker promotes lung cancer metastasis and progression.Biochim Biophys Acta. 2015 Jun;1854(6):668-76. doi: 10.1016/j.bbapap.2014.11.002. Epub 2014 Nov 14. Biochim Biophys Acta. 2015. PMID: 25463045 Clinical Trial.
-
Expression of Stanniocalcin 2 in Breast Cancer and Its Clinical Significance.Curr Med Sci. 2019 Dec;39(6):978-983. doi: 10.1007/s11596-019-2131-2. Epub 2019 Dec 16. Curr Med Sci. 2019. PMID: 31845230
-
STC2 Is a Potential Prognostic Biomarker for Pancreatic Cancer and Promotes Migration and Invasion by Inducing Epithelial-Mesenchymal Transition.Biomed Res Int. 2019 Jul 15;2019:8042489. doi: 10.1155/2019/8042489. eCollection 2019. Biomed Res Int. 2019. PMID: 32258098 Free PMC article.
-
New Insights Into Physiological and Pathophysiological Functions of Stanniocalcin 2.Front Endocrinol (Lausanne). 2020 Mar 31;11:172. doi: 10.3389/fendo.2020.00172. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32296395 Free PMC article. Review.
-
The significance of Stanniocalcin 2 in malignancies and mechanisms.Bioengineered. 2021 Dec;12(1):7276-7285. doi: 10.1080/21655979.2021.1977551. Bioengineered. 2021. PMID: 34612765 Free PMC article. Review.
Cited by
-
Stanniocalcin 2 governs cancer cell adaptation to nutrient insufficiency through alleviation of oxidative stress.Cell Death Dis. 2024 Aug 6;15(8):567. doi: 10.1038/s41419-024-06961-7. Cell Death Dis. 2024. PMID: 39107307 Free PMC article.
-
A gene expression-based classifier for HER2-low breast cancer.Sci Rep. 2024 Feb 1;14(1):2628. doi: 10.1038/s41598-024-52148-7. Sci Rep. 2024. PMID: 38297001 Free PMC article.
-
Identification and exploration of key genes associated with radioresistance in lung adenocarcinoma.Cancer Cell Int. 2025 Apr 19;25(1):155. doi: 10.1186/s12935-025-03783-1. Cancer Cell Int. 2025. PMID: 40253319 Free PMC article.
-
Molecular characterization of Golgi apparatus-related genes indicates prognosis and immune infiltration in osteosarcoma.Aging (Albany NY). 2024 Mar 7;16(6):5249-5263. doi: 10.18632/aging.205645. Epub 2024 Mar 7. Aging (Albany NY). 2024. PMID: 38460960 Free PMC article.
-
Pretreated exosomes by electrical stimulation accelerate bone regeneration.Bioact Mater. 2025 May 20;51:383-398. doi: 10.1016/j.bioactmat.2025.04.019. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40491687 Free PMC article.
References
-
- Stannius H. Über Nebennieren bei Knochenfischen. Arch Anat Physiol Wissenschaft Med. 1839;6:97–101.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

