A fast extraction-free isothermal LAMP assay for detection of SARS-CoV-2 with potential use in resource-limited settings
- PMID: 35501862
- PMCID: PMC9059459
- DOI: 10.1186/s12985-022-01800-7
A fast extraction-free isothermal LAMP assay for detection of SARS-CoV-2 with potential use in resource-limited settings
Abstract
Background: To retain the spread of SARS-CoV-2, fast, sensitive and cost-effective testing is essential, particularly in resource limited settings (RLS). Current standard nucleic acid-based RT-PCR assays, although highly sensitive and specific, require transportation of samples to specialised laboratories, trained staff and expensive reagents. The latter are often not readily available in low- and middle-income countries and this may significantly impact on the successful disease management in these settings. Various studies have suggested a SARS-CoV-2 loop mediated isothermal amplification (LAMP) assay as an alternative method to RT-PCR.
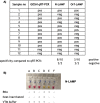
Methods: Four previously published primer pairs were used for detection of SARS-CoV-2 in the LAMP assay. To determine optimal conditions, different temperatures, sample input and incubation times were tested. Ninety-three extracted RNA samples from St. George's Hospital, London, 10 non-extracted nasopharyngeal swab samples from Great Ormond Street Hospital for Children, London, and 92 non-extracted samples from Queen Elisabeth Central Hospital (QECH), Malawi, which have previously been tested for SARS-Cov-2 by quantitative reverse-transcription RealTime PCR (qRT-PCR), were analysed in the LAMP assay.
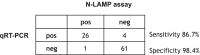
Results: In this study we report the optimisation of an extraction-free colourimetric SARS-CoV-2 LAMP assay and demonstrated that a lower limit of detection (LOD) between 10 and 100 copies/µL of SARS-CoV-2 could be readily detected by a colour change of the reaction within as little as 30 min. We further show that this assay could be quickly established in Malawi, as no expensive equipment is necessary. We tested 92 clinical samples from QECH and showed the sensitivity and specificity of the assay to be 86.7% and 98.4%, respectively. Some viral transport media, used routinely to stabilise RNA in clinical samples during transportation, caused a non-specific colour-change in the LAMP reaction and therefore we suggest collecting samples in phosphate buffered saline (which did not affect the colour) as the assay allows immediate sample analysis on-site.
Conclusion: SARS-CoV-2 LAMP is a cheap and reliable assay that can be readily employed in RLS to improve disease monitoring and management.
Keywords: Extraction-free LAMP; Resource-limited settings; SARS-CoV-2; qRT-PCR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests related to this work.
Figures


References
-
- John’s Hopkins University Coronavirus Resource Centre 2020 [Available from: https://coronavirus.jhu.edu.
-
- Institute of Viral Diseases. China CDC. National Institute for Viral Disease Control and Prevention 2020.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

