Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: a systematic review and meta-analysis
- PMID: 35501872
- PMCID: PMC9059401
- DOI: 10.1186/s13643-022-01950-3
Investigating the safety and efficacy of hematopoietic and mesenchymal stem cell transplantation for treatment of T1DM: a systematic review and meta-analysis
Abstract
Background: Stem cell transplantation (SCT) has paved the way for treatment of autoimmune diseases. SCT has been investigated in type 1 diabetes mellitus (T1DM) as an autoimmune-based disorder, but previous studies have not presented a comprehensive view of its effect on treatment of T1DM.
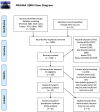
Methodology: After registration of the present systematic review and meta-analysis in the PROSPERO, a search was done according to the Cochrane guidelines for evaluation of clinical trials to find eligible clinical trials that investigated the effect of SCT on T1DM (based on ADA® diagnostic criteria) from PubMed, Web of science, Scopus, etc, as well as registries of clinical trials from January 1, 2000, to September 31, 2019. A search strategy was designed using MeSH and EM-tree terms. Primary outcome included the changes in the insulin total daily dose (TDD) (U/kg) level, and secondary outcomes included the changes in the HbA1c, c-peptide, and adjusted HbA1c levels. The Q Cochrane test and I2 statistic were performed to assess the heterogeneity and its severity in primary clinical trials. The Cochrane ROB was used to determine risk of bias, and Cochrane Handbook for Systematic Reviews of Interventions was used in the full text papers. The meta-analysis was accomplished in the STATA software, and the results were shown on their forest plots. Confounders were evaluated by the meta-regression test.
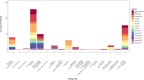
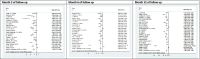
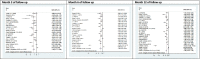
Results: A total of 9452 studies were electronically screened, and 35 papers were included for data extraction. The results of this review study showed that 173 (26.5%) diabetic patients experienced insulin-free period (from 1 to 80 months), and 445 (68%) showed reduction in TDD of insulin after the SCT. Combination of hematopoietic stem cell (HSC) with mesenchymal stem cell (MSC) transplantation were significantly associated with improvement of the TDD (SMD: - 0.586, 95% CI: - 1.204/- 0.509, I2: 0%), HbA1c (SMD: - 0.736, 95% CI: - 1.107/- 0.365, I2: 0%), adjusted HbA1c (SMD: - 2.041, 95% CI: - 2.648/- 1.434, I2: 38.4%), and c-peptide (SMD: 1.917, 95% CI: 0.192/3.641, I2: 92.5%) on month 3 of follow-up, while its association had a growing trend from 3 to 12 months after the transplantation. Considering severe adverse events, HSC transplantation accompanied with conditioning could not be suggested as a safe treatment.
Conclusion: Most of the clinical trials of SCT in T1DM were single arm. Although meta-analysis illustrated the SCT is associated with T1DM improvement, well-designed randomized clinical trials are needed to clarify its efficacy.
Recommendation: Based on the results of this meta-analysis, the MSC and its combination with HSC could be considered as "Safe Cell" for SCT in T1DM. Furthermore, to evaluate the SCT efficacy, calculation of insulin TDD (U/kg/day), AUC of c-peptide, and adjusted HbA1c are highly recommended.
Keywords: Cell transplantation; Clinical trial; Hematopoietic; Mesenchymal stem cell; Type 1 diabetes mellitus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Stem cell transplantation for induction of remission in medically refractory Crohn's disease.Cochrane Database Syst Rev. 2022 May 13;5(5):CD013070. doi: 10.1002/14651858.CD013070.pub2. Cochrane Database Syst Rev. 2022. PMID: 35556242 Free PMC article.
-
Home treatment for mental health problems: a systematic review.Health Technol Assess. 2001;5(15):1-139. doi: 10.3310/hta5150. Health Technol Assess. 2001. PMID: 11532236
-
Psychological and/or educational interventions for the prevention of depression in children and adolescents.Cochrane Database Syst Rev. 2004;(1):CD003380. doi: 10.1002/14651858.CD003380.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Dec 07;(12):CD003380. doi: 10.1002/14651858.CD003380.pub3. PMID: 14974014 Updated.
-
Interventions for promoting habitual exercise in people living with and beyond cancer.Cochrane Database Syst Rev. 2018 Sep 19;9(9):CD010192. doi: 10.1002/14651858.CD010192.pub3. Cochrane Database Syst Rev. 2018. PMID: 30229557 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
Cited by
-
Monitoring of Autoantibodies Following Autologous Hematopoietic Stem Cell Transplantation in 6 Children with Recently Diagnosed Type 1 Diabetes Mellitus.Med Sci Monit. 2023 Jan 20;29:e938979. doi: 10.12659/MSM.938979. Med Sci Monit. 2023. PMID: 36659834 Free PMC article.
-
Advancements in diabetes research and stem cell therapy: a concise review.J Diabetes Metab Disord. 2025 May 29;24(1):130. doi: 10.1007/s40200-025-01638-0. eCollection 2025 Jun. J Diabetes Metab Disord. 2025. PMID: 40454188 Review.
-
A Supportive Role of Mesenchymal Stem Cells on Insulin-Producing Langerhans Islets with a Specific Emphasis on The Secretome.Biomedicines. 2023 Sep 18;11(9):2558. doi: 10.3390/biomedicines11092558. Biomedicines. 2023. PMID: 37761001 Free PMC article. Review.
-
The Role of Biotechnology in Latest Therapeutic Approaches for Diabetes Mellitus.Avicenna J Med Biotechnol. 2024 Apr-Jun;16(2):66-67. doi: 10.18502/ajmb.v16i2.14854. Avicenna J Med Biotechnol. 2024. PMID: 38618507 Free PMC article. No abstract available.
-
New frontiers in type I diabetes treatment: the impact of mesenchymal stromal cells on long-term complications.Front Clin Diabetes Healthc. 2025 May 19;6:1586061. doi: 10.3389/fcdhc.2025.1586061. eCollection 2025. Front Clin Diabetes Healthc. 2025. PMID: 40458682 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

